Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition - PubMed (original) (raw)
Review
Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition
Dawn M Eagle et al. Neurosci Biobehav Rev. 2010 Jan.
Abstract
Many common psychiatric conditions, such as attention deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), Parkinson's disease, addiction and pathological gambling are linked by a failure in the mechanisms that control, or inhibit, inappropriate behavior. Models of rat behavioral inhibition permit us to study in detail the anatomical and pharmacological bases of inhibitory failure, using methods that translate directly with patient assessment in the clinic. This review updates current ideas relating to behavioral inhibition based on two significant lines of evidence from rat studies: (1) To integrate new findings from the stop-signal task into existing models of behavioral inhibition, in particular relating to 'impulsive action' control. The stop-signal task has been used for a number of years to evaluate psychiatric conditions and has recently been translated for use in the rat, bringing a wealth of new information to behavioral inhibition research. (2) To consider the importance of the subthalamic nucleus (STN) in the neural circuitry of behavioral inhibition. This function of this nucleus is central to a number of 'disinhibitory' disorders such as Parkinson's disease and OCD, and their therapies, but its role in behavioral inhibition is still undervalued, and often not considered in preclinical models of behavioral control. Integration of these findings has pinpointed the orbitofrontal cortex (OF), dorsomedial striatum (DMStr) and STN within a network that normally inhibits many forms of behavior, including both impulsive and compulsive forms. However, there are distinct differences between behavioral subtypes in their neurochemical modulation. This review brings new light to the classical view of the mechanisms that inhibit behavior, in particular suggesting a far more prominent role for the STN, a structure that is usually omitted from conventional behavioral-inhibition networks. The OF-DMStr-STN circuitry may form the basis of a control network that defines behavioral inhibition and that acts to suppress or countermand many forms of inappropriate or maladaptive behavior.
Figures
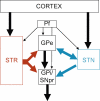
Fig. 1
Schematic representation of the model of the basal ganglia proposed by Levy et al. (1997). Str: Striatum; Pf: parafascicular nucleus of the thalamus; GPe: External segment of the Globus Pallidus; GPi: Internal segment of the Globus Pallidus; SNpr: Substantia nigra pars reticulata; STN: subthalamic nucleus.
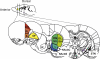
Fig. 2
Schematic sections of rat brain showing some of the regions of the cortex and basal ganglia that mediate the control of behavioral inhibition. CG: pre-genual cingulate cortex, PL: prelimbic cortex, IL: infralimbic cortex, OF: orbitofrontal cortex, DMStr: dorsomedial striatum, DLStr: dorsolateral striatum, NAcbC: nucleus accumbens core, NAcbS: nucleus accumbens shell, STN: subthalamic nucleus.
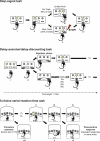
Fig. 3
Schematic representation of (a) the stop-signal task, (b) the delay-discounting task and (c) the 5-CSRT task. Each figure shows the functional panels from operant-conditioning chambers (a and b) and the 9-hole, or 5-hole box (c). In the stop-signal task, rats begin each trial with a nose poke in the central food magazine (i). The go trial phase begins with a left lever press (ii) and then the rat must move quickly to press the right lever (iii) to complete the ‘go’ response. A correct trial is rewarded with a food pellet (iv). On 20% of trials (randomly distributed through the session), a stop signal during the go phase signals that the rat must inhibit the right lever press (v) to receive a food pellet. In the delay-aversion/delay-discounting task, trials begin automatically (i) with presentation of both levers (ii). Selection of one lever (e.g., the left lever, iii) gives one food pellet with no delay (iv). Selection of the other lever (e.g., the right lever (v) gives four pellets but after a delay of 0, 10, 20, 40, or 60 s (vi). The rats receive an inter-trial interval (ITI) for the remainder of each 100-s trial to ensure that rats completing no-delay trials do not earn greater numbers of rewards simply by completing greater numbers of trials. In the 5-CSRT task, the rat begins each trial with a nose poke in the food magazine (i), which is located on the opposite wall of the chamber to the response apertures. Following a 5-second ITI a brief (500ms) light appears in one of the apertures (ii) and the rat must make a nose poke response in that hole (iii) to receive a food reward (iv). Responding in a different hole is incorrect (v). Perseverative responding is measured as repeated nose poke responses after the food has been delivered (vi). Impulsive action is measured as premature responses, where a response occurs during the ITI (vii) before the light signal.
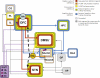
Fig. 4
Schematic representation of the major inhibitory processes in the stop-signal, 5-CSRT and delay-discounting tasks from lesion and pharmacological manipulations. Colored bands surrounding each structure highlight the roles of key structures in inhibitory processes. Hatched-shaded bands indicates no effect of excitotoxic lesions but an effect under other circumstances (e.g., dependent on previous behavior, or following pharmacological manipulations). Grey structures indicate that no information is available. Arrows highlight connections between regions that are of interest for response inhibition networks.
Similar articles
- Inhibition and impulsivity: behavioral and neural basis of response control.
Bari A, Robbins TW. Bari A, et al. Prog Neurobiol. 2013 Sep;108:44-79. doi: 10.1016/j.pneurobio.2013.06.005. Epub 2013 Jul 13. Prog Neurobiol. 2013. PMID: 23856628 Review. - Deep-Brain Stimulation of the Subthalamic Nucleus Selectively Decreases Risky Choice in Risk-Preferring Rats.
Adams WK, Vonder Haar C, Tremblay M, Cocker PJ, Silveira MM, Kaur S, Baunez C, Winstanley CA. Adams WK, et al. eNeuro. 2017 Aug 7;4(4):ENEURO.0094-17.2017. doi: 10.1523/ENEURO.0094-17.2017. eCollection 2017 Jul-Aug. eNeuro. 2017. PMID: 28791332 Free PMC article. - High frequency stimulation and temporary inactivation of the subthalamic nucleus reduce quinpirole-induced compulsive checking behavior in rats.
Winter C, Mundt A, Jalali R, Joel D, Harnack D, Morgenstern R, Juckel G, Kupsch A. Winter C, et al. Exp Neurol. 2008 Mar;210(1):217-28. doi: 10.1016/j.expneurol.2007.10.020. Epub 2007 Nov 17. Exp Neurol. 2008. PMID: 18076877 - Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology.
Korff S, Harvey BH. Korff S, et al. Psychiatr Clin North Am. 2006 Jun;29(2):371-90. doi: 10.1016/j.psc.2006.02.007. Psychiatr Clin North Am. 2006. PMID: 16650714 Review.
Cited by
- Social context and drug cues modulate inhibitory control in cocaine addiction: involvement of the STN evidenced through functional MRI.
Terenzi D, Simon N, Gachomba MJM, de Peretti JL, Nazarian B, Sein J, Anton JL, Grandjean D, Baunez C, Chaminade T. Terenzi D, et al. Mol Psychiatry. 2024 Dec;29(12):3742-3751. doi: 10.1038/s41380-024-02637-y. Epub 2024 Jun 26. Mol Psychiatry. 2024. PMID: 38926543 Free PMC article. - Chemogenetic Activation of Midbrain Dopamine Neurons Affects Attention, but not Impulsivity, in the Five-Choice Serial Reaction Time Task in Rats.
Boekhoudt L, Voets ES, Flores-Dourojeanni JP, Luijendijk MC, Vanderschuren LJ, Adan RA. Boekhoudt L, et al. Neuropsychopharmacology. 2017 May;42(6):1315-1325. doi: 10.1038/npp.2016.235. Epub 2016 Oct 17. Neuropsychopharmacology. 2017. PMID: 27748741 Free PMC article. - Reactive and Proactive Adaptation of Cognitive and Motor Neural Signals during Performance of a Stop-Change Task.
Brockett AT, Roesch MR. Brockett AT, et al. Brain Sci. 2021 May 11;11(5):617. doi: 10.3390/brainsci11050617. Brain Sci. 2021. PMID: 34064876 Free PMC article. Review. - Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction.
Jupp B, Caprioli D, Dalley JW. Jupp B, et al. Dis Model Mech. 2013 Mar;6(2):302-11. doi: 10.1242/dmm.010934. Epub 2013 Jan 25. Dis Model Mech. 2013. PMID: 23355644 Free PMC article. Review. - Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease.
Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WP. Wylie SA, et al. Brain. 2010 Dec;133(Pt 12):3611-24. doi: 10.1093/brain/awq239. Epub 2010 Sep 22. Brain. 2010. PMID: 20861152 Free PMC article.
References
- Amalric M., Koob G.F. Dorsal pallidum as a functional motor output of the corpus striatum. Brain Res. 1989;483:389–394. - PubMed
- Amalric M., Moukhles H., Nieoullon A., Daszuta A. Complex deficits on reaction time performance following bilateral intrastriatal 6-OHDA infusion in the rat. Eur. J. Neurosci. 1995;7:972–980. - PubMed
- Aron A.R. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. - PubMed
- Aron A.R., Dowson J.H., Sahakian B.J., Robbins T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2003;54:1465–1468. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials