Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus - PubMed (original) (raw)
Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus
Xiang Gao et al. Exp Neurol. 2009 Oct.
Abstract
Recent evidence shows that traumatic brain injury (TBI) regulates proliferation of neural stem/progenitor cells in the dentate gyrus (DG) of adult hippocampus. There are distinct classes of neural stem/progenitor cells in the adult DG, including quiescent neural progenitors (QNPs), which carry stem cell properties, and their progeny, amplifying neural progenitors (ANPs). The response of each class of progenitors to TBI is not clear. We here used a transgenic reporter Nestin-GFP mouse line, in which QNP and ANP cells are easily visualized and quantified, to determine the targets of the TBI in the DG. We examined changes in proliferation of QNPs and ANPs in the acute phase following TBI and found that QNPs were induced by TBI insult to enter the cell cycle whereas proliferation of ANPs was not significantly affected. These results indicate that different subtypes of neural stem/progenitor cells respond differently to TBI insult. Stem cell activation by the TBI may reflect the induction of innate repair and plasticity mechanisms by the injured brain.
Figures
Fig. 1
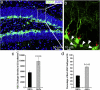
TBI insult promotes cell proliferation in the adult hippocampus. (a, b) Immunostaining with antibody against BrdU (red) to identify proliferating cells in the hippocampus of sham-operated mice (a, b) or of mice after TBI surgery (c, d). Nuclei are stained with DAPI (blue) to show the DG structure. Quantification shows the distribution of proliferating cells in the different subregions of hippocampus. ML: molecular layer; GCL: granule cell layer. SGZ: subgranular zone.
Fig. 2
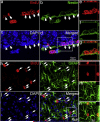
Determining the cell types the proliferating cells in the hippocampus following moderate TBI. (a–g) Double immunostaining with antibody against BrdU (red) and Nestin (green) was performed to visualize the proliferating neural stem/progenitor cells in the SGZ of the hippocampus after TBI. (a) BrdU. (b) Nestin. (c) DAPI. (d) Merge of (a) to (c). White arrows indicate proliferating neural stem/progenitor cells. (e–g) Confocal microscopy was performed to verify the colocalization of BrdU with Nestin in the cells within the white box in panel (d). (h–n) Double immunostaining with antibody against BrdU (red) and GFAP (green) was performed to show the reactive astrocytes in the ML of the hippocampus after TBI. (h) BrdU. (i) GFAP. (j) DAPI. (k) Merge of (h) to (j). White arrows indicate the reactive astrocytes. (e–g) Confocal microscopy was performed to verify the colocalization of BrdU with GFAP in the cells within the white box in panel (k).
Fig. 3
Quiescent neural progenitors (QNPs) and amplifying neural progenitors (ANPs) are distinguishable in the hippocampus of Nestin-EGFP transgenic mice. (a, b) EGFP immunostaining was performed to reveal the QNPs and ANPs in the adult hippocampus of Nestin-EGFP transgenic mice. QNPs are marked by white arrows, while the ANPs are marked by white arrowheads. (c, d). Quantification of QNPs and ANPs in the adult hippocampus of Nestin-EGFP transgenic mice.
Fig. 4
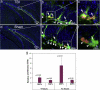
Assessing the proliferation of QNPs and ANPs in the hippocampus following moderate TBI. Double immunostainings were performed to reveal the proliferating QNPs and ANPs in the adult hippocampus with antibodies against EGFP and BrdU. (a–c). Proliferation of QNPs and ANPs in the adult hippocampus of moderate CCI-injured mice. (d–f). Proliferation of QNPs and ANPs in the adult hippocampus of moderate CCI-injured mice. Nuclei are stained with DAPI (blue) to show the DG structure. QNPs are marked by white arrows, while the ANPs are marked by white arrowheads. (g) The proliferation rates of QNP and ANP were obtained in CCI-injured mice and control mice. Their relative proliferation rates in fold were calculated by comparing the proliferation rates in CCI-injured mice to control mice.
Similar articles
- Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors.
Yu TS, Zhang G, Liebl DJ, Kernie SG. Yu TS, et al. J Neurosci. 2008 Nov 26;28(48):12901-12. doi: 10.1523/JNEUROSCI.4629-08.2008. J Neurosci. 2008. PMID: 19036984 Free PMC article. - Chronic Lithium Treatment Enhances the Number of Quiescent Neural Progenitors but Not the Number of DCX-Positive Immature Neurons.
Kara N, Narayanan S, Belmaker RH, Einat H, Vaidya VA, Agam G. Kara N, et al. Int J Neuropsychopharmacol. 2015 Jan 29;18(7):pyv003. doi: 10.1093/ijnp/pyv003. Int J Neuropsychopharmacol. 2015. PMID: 25636892 Free PMC article. - Neural stem and progenitor cells in nestin-GFP transgenic mice.
Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Mignone JL, et al. J Comp Neurol. 2004 Feb 9;469(3):311-24. doi: 10.1002/cne.10964. J Comp Neurol. 2004. PMID: 14730584 - Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis.
Amiri A, Cho W, Zhou J, Birnbaum SG, Sinton CM, McKay RM, Parada LF. Amiri A, et al. J Neurosci. 2012 Apr 25;32(17):5880-90. doi: 10.1523/JNEUROSCI.5462-11.2012. J Neurosci. 2012. PMID: 22539849 Free PMC article. - Neurogenesis after traumatic brain injury.
Richardson RM, Sun D, Bullock MR. Richardson RM, et al. Neurosurg Clin N Am. 2007 Jan;18(1):169-81, xi. doi: 10.1016/j.nec.2006.10.007. Neurosurg Clin N Am. 2007. PMID: 17244562 Review.
Cited by
- Current advances in neurotrauma research: diagnosis, neuroprotection, and neurorepair.
Chen J, Shi R. Chen J, et al. Neural Regen Res. 2014 Jun 1;9(11):1093-5. doi: 10.4103/1673-5374.135306. Neural Regen Res. 2014. PMID: 25206764 Free PMC article. No abstract available. - Post-Injury Treatment of 7,8-Dihydroxyflavone Promotes Neurogenesis in the Hippocampus of the Adult Mouse.
Zhao S, Yu A, Wang X, Gao X, Chen J. Zhao S, et al. J Neurotrauma. 2016 Nov 15;33(22):2055-2064. doi: 10.1089/neu.2015.4036. Epub 2016 Apr 28. J Neurotrauma. 2016. PMID: 26715291 Free PMC article. - Combination treatment with ethyl pyruvate and IGF-I exerts neuroprotective effects against brain injury in a rat model of neonatal hypoxic-ischemic encephalopathy.
Rong Z, Pan R, Chang L, Lee W. Rong Z, et al. Int J Mol Med. 2015 Jul;36(1):195-203. doi: 10.3892/ijmm.2015.2219. Epub 2015 May 22. Int J Mol Med. 2015. PMID: 25999282 Free PMC article. - Therapeutic Application of Stem Cells in the Repair of Traumatic Brain Injury.
Adugna DG, Aragie H, Kibret AA, Belay DG. Adugna DG, et al. Stem Cells Cloning. 2022 Jul 13;15:53-61. doi: 10.2147/SCCAA.S369577. eCollection 2022. Stem Cells Cloning. 2022. PMID: 35859889 Free PMC article. Review. - Differential Response in Novel Stem Cell Niches of the Brain after Cervical Spinal Cord Injury and Traumatic Brain Injury.
Falnikar A, Stratton J, Lin R, Andrews CE, Tyburski A, Trovillion VA, Gottschalk C, Ghosh B, Iacovitti L, Elliott MB, Lepore AC. Falnikar A, et al. J Neurotrauma. 2018 Sep 15;35(18):2195-2207. doi: 10.1089/neu.2017.5497. Epub 2018 Jun 7. J Neurotrauma. 2018. PMID: 29471717 Free PMC article.
References
- Ariza M, Serra-Grabulosa JM, Junque C, Ramirez B, Mataro M, Poca A, Bargallo N, Sahuquillo J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. - PubMed
- Bhattacharjee Y. Neuroscience. Shell shock revisited: solving the puzzle of blast trauma. Science. 2008;319:406–408. - PubMed
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources