Deletion of the p107/p130-binding domain of Mip130/LIN-9 bypasses the requirement for CDK4 activity for the dissociation of Mip130/LIN-9 from p107/p130-E2F4 complex - PubMed (original) (raw)
Deletion of the p107/p130-binding domain of Mip130/LIN-9 bypasses the requirement for CDK4 activity for the dissociation of Mip130/LIN-9 from p107/p130-E2F4 complex
Raudel Sandoval et al. Exp Cell Res. 2009.
Abstract
Mip130/LIN-9 is part of a large complex that includes homologs of the Drosophila dREAM (drosophila RB-like, E2F, and Myb) and C. elegans DRM complexes. This complex also includes proteins such as Mip40/LIN-37, Mip120/LIN-54, and LIN-52. In mammalian cells, Mip130/LIN-9 specifically associates with the p107/p130-E2F4 repressor complex in G0/G1 and with B-Myb in S-phase. However, little is known about how the transition occurs and whether Mip130/LIN-9 contributes to the repressor effect of p107/p130. In this report, we demonstrate that Mip130/LIN-9, Mip40/LIN-37, Mip120/LIN-54, and Sin3b form a core complex, the Mip Core Complex or LIN Complex (MCC/LINC), which is detectable in all phases of the cell cycle. This complex specifically recruits transcriptional repressors such as p107, p130, E2F4 and HDAC1 in G0/G1, and B-Myb in S-phase. Importantly, we provide strong evidence that the transition between repressors and activators of transcription is mediated by CDK4, through the phosphorylation of the pocket proteins, p107 and p130. The requirement for CDK4 activity is bypassed by the deletion of the first 84 amino acids (Mip130/LIN-9(Delta84)), since this mutant is unable to interact with p107/p130 in G0/G1, while maintaining its association with B-Myb. Importantly, the Mip130/LIN-9(Delta84) allele rescues the low expression of G1/S genes observed in CDK4(-/-) MEFs demonstrating that Mip130/LIN-9 contributes to the repression of these E2F-regulated genes in G0/G1.
Figures
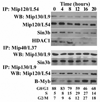
Figure 1. The mammalian orthologs of the dREAM complex form a core complex that includes Sin3b
Serum-starved NIH/3T3 cells were released by the addition of serum, and samples for FACS analysis and protein studies were collected every 4 hours. The cell cycle distribution is shown at the bottom. Nuclear extracts were used for immunoprecipitations (IP) with the indicated antibodies followed by immunoblotting (WB) with antibodies against the indicated mammalian orthologs of the dREAM complex proteins.
Figure 2. The MCC/LINC interacts with p107 in G0/G1 and B-Myb in S-phase
Protein lysates from the experiment described in Figure 1 were used for immunoprecipitations (IP) with anti-p107 (A) or -B-Myb (B) followed by immunoblotting (WB) with the indicated antibodies. The interactions of B-Myb and p107 with Mip40/LIN-37 were studied by performing immunoprecipitations with the anti-Mip40/LIN-37 serum since the reciprocal experiments cannot be performed due to the close migration of Mip40/LIN-37 to the IgG. The presence of a background band occasionally observed in B-Myb immunoblots is indicated.
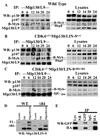
Figure 3. CDK4 drives the separation of Mip/LIN-9 from p107/p130
Synchronized MEFs derived from wild type (A), CDK4−/− (B), or CDK4−/−Mip130/LIN-9Δ84/Δ84 (C) animals were used for immunoprecipitations with an anti-Mip130/LIN-9 mAb followed by immunoblotting (WB) with the indicated antibodies (A–C, left panels). In the right panels (A–C), 30 µg of nuclear lysate (10% of the input used in the immunoprecipitations) were processed in parallel to determine the total amount of the different proteins expressed. D. On the left, protein lysates obtained from wild type (WT) or Mip130/LIN-9Δ84 (Δ84) cells were used for 1immunoprecipitations with the anti-Mip120/LIN-54 serum followed by immunoblotting with the anti-Mip130/LIN-9 mAb. The full-length Mip130/LIN-9 and Mip130/LIN-9 Δ84 are noted as FL and Δ84, respectively. E. 293FT cells were transfected with GFP-Mip130/LIN-9Δ84 and HAMip40/ LIN-37 and immunoprecipitations were performed with anti-GFP or -HA antibodies followed by immunoblotting with the indicated antibodies. Immunoprecipitations with normal rabbit serum (NR) and monoclonal mouse IgG (IgG) were used as controls.
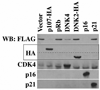
Figure 4. The inhibition of CDK4 or the overexpression of p107 leads to the degradation of B-Myb
T98G cells were transfected with equal amounts of the indicated recombinant constructs and FLAG- tagged B-Myb (10 µg of total plasmid DNA) for 24 hours. Cell lysates (50 µg) were directly analyzed by SDS-PAGE and immunoblotted (WB) first with the FLAG (M2) mAb to assess the expression of FLAG-tagged B-Myb. Subsequent immunoblotting was then performed with the indicated antibodies to verify the transfection of the appropriate recombinant constructs.
Figure 5. The Mip130/LIN-9Δ84 allele rescues the defect in the transcription of G1/S genes present in CDK4-null cells
Wild-type, CDK4−/−, and CDK4−/−Mip130/LIN-9Δ84/Δ84 cells were synchronized by serum starvation and released in growth medium for the indicated times. Total RNA was extracted and RT-PCR was performed as described in Materials and Methods. Expression differences were calculated as previously described [31]. PCR reactions were run in triplicates and experiments were repeated 2–3 times. Of note, no rescue was observed in B-Myb transcription.
Similar articles
- Mammalian Mip/LIN-9 interacts with either the p107, p130/E2F4 repressor complex or B-Myb in a cell cycle-phase-dependent context distinct from the Drosophila dREAM complex.
Pilkinton M, Sandoval R, Colamonici OR. Pilkinton M, et al. Oncogene. 2007 Nov 29;26(54):7535-43. doi: 10.1038/sj.onc.1210562. Epub 2007 Jun 11. Oncogene. 2007. PMID: 17563750 - Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence.
Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA. Litovchick L, et al. Mol Cell. 2007 May 25;26(4):539-51. doi: 10.1016/j.molcel.2007.04.015. Mol Cell. 2007. PMID: 17531812 - E2F/p107 and E2F/p130 complexes are regulated by C/EBPalpha in 3T3-L1 adipocytes.
Timchenko NA, Wilde M, Iakova P, Albrecht JH, Darlington GJ. Timchenko NA, et al. Nucleic Acids Res. 1999 Sep 1;27(17):3621-30. doi: 10.1093/nar/27.17.3621. Nucleic Acids Res. 1999. PMID: 10446255 Free PMC article. - TGFbeta-mediated formation of pRb-E2F complexes in human myeloid leukemia cells.
Hu XT. Hu XT. Biochem Biophys Res Commun. 2008 May 2;369(2):277-80. doi: 10.1016/j.bbrc.2008.02.051. Epub 2008 Feb 21. Biochem Biophys Res Commun. 2008. PMID: 18294958 Review. - The p130 pocket protein: keeping order at cell cycle exit/re-entrance transitions.
Mayol X, Grana X. Mayol X, et al. Front Biosci. 1998 Jan 1;3:d11-24. doi: 10.2741/a263. Front Biosci. 1998. PMID: 9405335 Review.
Cited by
- Drosophila RB proteins repress differentiation-specific genes via two different mechanisms.
Lee H, Ohno K, Voskoboynik Y, Ragusano L, Martinez A, Dimova DK. Lee H, et al. Mol Cell Biol. 2010 May;30(10):2563-77. doi: 10.1128/MCB.01075-09. Epub 2010 Feb 22. Mol Cell Biol. 2010. PMID: 20176807 Free PMC article. - Myb proteins: angels and demons in normal and transformed cells.
Zhou Y, Ness SA. Zhou Y, et al. Front Biosci (Landmark Ed). 2011 Jan 1;16(3):1109-31. doi: 10.2741/3738. Front Biosci (Landmark Ed). 2011. PMID: 21196221 Free PMC article. Review. - The potential of targeting Sin3B and its associated complexes for cancer therapy.
Cantor DJ, David G. Cantor DJ, et al. Expert Opin Ther Targets. 2017 Nov;21(11):1051-1061. doi: 10.1080/14728222.2017.1386655. Epub 2017 Oct 9. Expert Opin Ther Targets. 2017. PMID: 28956957 Free PMC article. Review. - The HDAC-Associated Sin3B Protein Represses DREAM Complex Targets and Cooperates with APC/C to Promote Quiescence.
Bainor AJ, Saini S, Calderon A, Casado-Polanco R, Giner-Ramirez B, Moncada C, Cantor DJ, Ernlund A, Litovchick L, David G. Bainor AJ, et al. Cell Rep. 2018 Dec 4;25(10):2797-2807.e8. doi: 10.1016/j.celrep.2018.11.024. Cell Rep. 2018. PMID: 30517867 Free PMC article. - The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation.
Soppa U, Schumacher J, Florencio Ortiz V, Pasqualon T, Tejedor FJ, Becker W. Soppa U, et al. Cell Cycle. 2014;13(13):2084-100. doi: 10.4161/cc.29104. Epub 2014 May 7. Cell Cycle. 2014. PMID: 24806449 Free PMC article.
References
- Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. - PubMed
- Ciemerych MA, Sicinski P. Cell cycle in mouse development. Oncogene. 2005;24:2877–2898. - PubMed
- Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. - PubMed
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. - PubMed
- van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol. 2008;9:713–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007739/DK/NIDDK NIH HHS/United States
- T32 DK07739/DK/NIDDK NIH HHS/United States
- R01 GM081562/GM/NIGMS NIH HHS/United States
- R01 GM-54709/GM/NIGMS NIH HHS/United States
- K01 CA127862/CA/NCI NIH HHS/United States
- R01 GM054709/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous