Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals - PubMed (original) (raw)
Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals
Scott Siechen et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Memory and learning in animals are mediated by neurotransmitters that are released from vesicles clustered at the synapse. As a synapse is used more frequently, its neurotransmission efficiency increases, partly because of increased vesicle clustering in the presynaptic neuron. Vesicle clustering has been believed to result primarily from biochemical signaling processes that require the connectivity of the presynaptic terminal with the cell body, the central nervous system, and the postsynaptic cell. Our in vivo experiments on the embryonic Drosophila nervous system show that vesicle clustering at the neuromuscular presynaptic terminal depends on mechanical tension within the axons. Vesicle clustering vanishes upon severing the axon from the cell body, but is restored when mechanical tension is applied to the severed end of the axon. Clustering increases when intact axons are stretched mechanically by pulling the postsynaptic muscle. Using micro mechanical force sensors, we find that embryonic axons that have formed neuromuscular junctions maintain a rest tension of approximately 1 nanonewton. If the rest tension is perturbed mechanically, axons restore the rest tension either by relaxing or by contracting over a period of approximately 15 min. Our results suggest that neuromuscular synapses employ mechanical tension as a signal to modulate vesicle accumulation and synaptic plasticity.
Figures
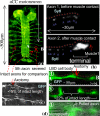
Fig. 1.
Synapse between aCC motoneuron and muscle1. (A) Embryonic nervous system of Drosophila (GFP) with aCC motoneurons. The figure also shows the axon that is severed in this study and the axons that are used for comparison. (B) Immunocytochemistry of the aCC axon with Synaptotagmin-I antibodies. Before the aCC contacts muscle1 (hour 13), Synaptotagmin-I is detected (blue arrowheads) along its axon (red dots). Soon after their initial contact (hour 14), Synaptotagmin-I (blue arrowheads) starts to exhibit distinct patterns of accumulation at the aCC axon terminal (bracket). The left end of the figure is the location of the LBD (lateral bipolar dendrite) cell and the location of the axotomy. (C) Axotomy of an axon before synaptogenesis. Here, the gap between the severed ends of the axon is small, <5% of the original length of the axon. (D) Axotomy of a typical axon AB (see A) after synaptogenesis. Now the gap between the cut ends is large, ≈22% of the original axon's length. The slack axon is pulled by a micropipette by holding the severed end with suction and moving the pipette with a stage. The motion is just enough to straighten the slack axon.
Fig. 2.
Force-modulated presynaptic assembly in axons severed by axotomy. (A) SytI comparison between severed and intact axons before synaptogenesis. We take an embryo after ≈12 h of embryogenesis (2 h before axon-muscle contact). We severed the fifth right-side axon from the posterior end of the embryo. After 2 h, we dissected the embryo and stained for SytI. We compared this severed axon with the intact fifth left axon (#5a) or the fourth right axon of the same embryo depending on the quality of imaging. The figure shows 6 axons, 3 of which were severed, the rest were left intact. The GFP images for 2 axons are shown to identify their shapes. The rest of the images show SytI along the length and at the terminal. The left end of the figure is the location of the LBD (lateral bipolar dendrite) cell and the location of the axotomy (see Fig. 1). The length of the axons shown is ≈30–40 μm. We found that severed axons find their muscle targets just as their intact neighbors. However, the axotomy decreases SytI accumulation at the aCC axon terminal. The bar chart and the plot compare 29 pairs of severed and intact axons from 29 embryos. The plot shows the average vesicle density (29 severed and 29 intact axons) along the length of the axon up to the terminal. The x axis shows the normalized lengths of the axons from the LBD (left end) to the terminal (right end) (see also Image analysis in
SI Text
). The variability among the axons is shown by the standard deviation bars. (B) SytI comparison between the severed axons after synaptogenesis, 1 pulled, the other unpulled. We take 2 embryos at the same stage of development, within ≈30 min of synaptogenesis (∼hr 14 of embryogenesis). The pair of embryos is placed in the same solution and on the same microscope slide. In each embryo, we severed the fifth axon from the posterior end on the right side of the embryo, pulled the cut axon of 1 of the embryos using a micropipette within 10 min of severing, leaving the other unpulled. The pull was held for 2 h, after which both of the embryos were fixed and stained for SytI. Thirteen such pairs were imaged. Eight of such axon samples are shown with SytI. The GFP images for 2 axons are shown to identify their shapes. Restoring the tension at the nascent synapse allows Synaptotagmin-I to accumulate at the terminal (data from 13 pairs).
Fig. 3.
Rest tension in the axon and its self regulation. (A) Scanning electron micrograph of a micro mechanical force sensor (spring constant, k = 3.5 nN/μ_m_) used to measure the force response of embryonic Drosophila axons. An x-y-z piezo stage held the sensor and brought the probe into contact with the axon to form nonspecific adhesion and apply stretch. The force, F = kx, on the probe was measured from the deflection, x, of the force sensing beams. The tension, T, in the axon was obtained from the force balance at the point of contact. (B) We dissected an embryo (after ≈16 h of embryogenesis) and removed the fat cells from around the fifth axon (from the posterior end of the embryo). We then used the probe to stretch the axon and measure its tension. The stretch was measured from geometry. The interval between the 2 data points was 50 s. Extrapolation of the force-stretch curve to 0 stretch point gave an estimate of the rest tension of ≈1 nN. (C) A probe pushed an axon at mid length in <1 s, and then held the stretch with time. The corresponding tension in the axon was measured as a function of time. (D) The probe was quickly released from a similarly stretched axon after its tension had relaxed to the rest value. The axon was overstretched as soon as the probe was removed. The time lapse images of the axon show that it shortened its length with time linearly with a velocity of 5 nm/s. The axon recovered its initial length in ≈10 min. _R_2 values of the linear fits in B and D are shown.
Fig. 4.
Force-modulated presynaptic assembly in axons. (A) A micro mechanical force probe contacts the muscle near the synapse in a wild-type embryo (≈16 h after embryogenesis) after fillet dissection, and pulls it, thus stretching the axon along its length (∼ 5% of its initial length). The axon was not subject to axotomy. The stretch was held for 90 min. The embryo was then stained for SytI. (B) We found that SytI accumulation in the pulled terminals was >2-fold higher compared with that in the unpulled neighboring terminals (data from 6 pairs, 100% implies the average value for the unpulled synapse).
Similar articles
- [Vesicle cycle in the presynaptic nerve terminal].
Zefirov AL. Zefirov AL. Ross Fiziol Zh Im I M Sechenova. 2007 May;93(5):544-62. Ross Fiziol Zh Im I M Sechenova. 2007. PMID: 17650622 Russian. - Vesicle turnover in developing neurons: how to build a presynaptic terminal.
Matteoli M, Coco S, Schenk U, Verderio C. Matteoli M, et al. Trends Cell Biol. 2004 Mar;14(3):133-40. doi: 10.1016/j.tcb.2004.01.007. Trends Cell Biol. 2004. PMID: 15003622 Review. - [Research progress of synaptic vesicle recycling].
Li YF, Zhang XX, Duan SM. Li YF, et al. Sheng Li Xue Bao. 2015 Dec 25;67(6):545-60. Sheng Li Xue Bao. 2015. PMID: 26701630 Review. Chinese. - Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity.
Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Mantilla CB, et al. Neuroscience. 2007 Apr 25;146(1):178-89. doi: 10.1016/j.neuroscience.2007.01.048. Epub 2007 Mar 7. Neuroscience. 2007. PMID: 17346898 - Relax? Don't do it!-Linking presynaptic vesicle clustering with mechanical tension.
Engerer P, Sigrist SJ. Engerer P, et al. HFSP J. 2009 Dec;3(6):367-72. doi: 10.2976/1.3260842. Epub 2009 Dec 10. HFSP J. 2009. PMID: 20514128 Free PMC article.
Cited by
- Mechanical control of tissue and organ development.
Mammoto T, Ingber DE. Mammoto T, et al. Development. 2010 May;137(9):1407-20. doi: 10.1242/dev.024166. Development. 2010. PMID: 20388652 Free PMC article. Review. - The emerging role of forces in axonal elongation.
Suter DM, Miller KE. Suter DM, et al. Prog Neurobiol. 2011 Jul;94(2):91-101. doi: 10.1016/j.pneurobio.2011.04.002. Epub 2011 Apr 20. Prog Neurobiol. 2011. PMID: 21527310 Free PMC article. Review. - Axon stretch growth: the mechanotransduction of neuronal growth.
Loverde JR, Tolentino RE, Pfister BJ. Loverde JR, et al. J Vis Exp. 2011 Aug 10;(54):2753. doi: 10.3791/2753. J Vis Exp. 2011. PMID: 21860373 Free PMC article. - Bacterial immobilization for imaging by atomic force microscopy.
Allison DP, Sullivan CJ, Mortensen NP, Retterer ST, Doktycz M. Allison DP, et al. J Vis Exp. 2011 Aug 10;(54):2880. doi: 10.3791/2880. J Vis Exp. 2011. PMID: 21860374 Free PMC article. - MEMS device for applying shear and tension to an epithelium combined with fluorescent live cell imaging.
Garcia MA, Sadeghipour E, Engel L, Nelson WJ, Pruitt BL. Garcia MA, et al. J Micromech Microeng. 2020;30(12):125004. doi: 10.1088/1361-6439/abb12c. Epub 2020 Oct 15. J Micromech Microeng. 2020. PMID: 34413578 Free PMC article.
References
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw Hill; 2000.
- Hebb D. Organization of behavior. New York: Wiley; 1949.
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. - PubMed
- Li Z, Sheng M. Some assembly required: The development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4:833–841. - PubMed
- Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1991;1:113–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials