The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration - PubMed (original) (raw)
Guideline
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration
Alessandro Liberati et al. BMJ. 2009.
Abstract
Systematic reviews and meta-analyses are essential to summarise evidence relating to efficacy and safety of healthcare interventions accurately and reliably. The clarity and transparency of these reports, however, are not optimal. Poor reporting of systematic reviews diminishes their value to clinicians, policy makers, and other users. Since the development of the QUOROM (quality of reporting of meta-analysis) statement-a reporting guideline published in 1999-there have been several conceptual, methodological, and practical advances regarding the conduct and reporting of systematic reviews and meta-analyses. Also, reviews of published systematic reviews have found that key information about these studies is often poorly reported. Realising these issues, an international group that included experienced authors and methodologists developed PRISMA (preferred reporting items for systematic reviews and meta-analyses) as an evolution of the original QUOROM guideline for systematic reviews and meta-analyses of evaluations of health care interventions. The PRISMA statement consists of a 27-item checklist and a four-phase flow diagram. The checklist includes items deemed essential for transparent reporting of a systematic review. In this explanation and elaboration document, we explain the meaning and rationale for each checklist item. For each item, we include an example of good reporting and, where possible, references to relevant empirical studies and methodological literature. The PRISMA statement, this document, and the associated website (www.prisma-statement.org/) should be helpful resources to improve reporting of systematic reviews and meta-analyses.
Conflict of interest statement
Competing interests: None declared.
Figures
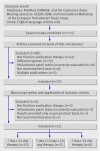
Fig 1 Flow of information through the different phases of a systematic review.
Fig 2 Example flow diagram of study selection. DDW = Digestive Disease Week; UEGW = United European Gastroenterology Week. Adapted from Fuccio et al
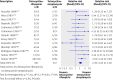
Fig 3 Example of summary results: Overall failure (defined as failure of assigned regimen or relapse) with tetracycline-rifampicin versus tetracycline-streptomycin. Adapted from Skalsky et al
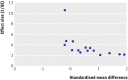
Fig 4 Example of a funnel plot showing evidence of considerable asymmetry. SE = standard error. Adapted from Appleton et al
Similar articles
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. Epub 2009 Jul 23. J Clin Epidemiol. 2009. PMID: 19631507 - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. Ann Intern Med. 2009 Aug 18;151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136. Epub 2009 Jul 20. Ann Intern Med. 2009. PMID: 19622512 - The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. Liberati A, et al. PLoS Med. 2009 Jul 21;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. Epub 2009 Jul 21. PLoS Med. 2009. PMID: 19621070 Free PMC article. - Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration.
Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, Tugwell P; PRISMA-Equity Bellagio group. Welch V, et al. J Clin Epidemiol. 2016 Feb;70:68-89. doi: 10.1016/j.jclinepi.2015.09.001. Epub 2015 Sep 5. J Clin Epidemiol. 2016. PMID: 26348799 - The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. Hutton B, et al. Ann Intern Med. 2015 Jun 2;162(11):777-84. doi: 10.7326/M14-2385. Ann Intern Med. 2015. PMID: 26030634
Cited by
- The effect and safety of corticosteroid treatment for severe community-acquired pneumonia: a meta-analysis of randomized controlled trials.
Chen Y, Kuang H, Zhu Y, Luo X. Chen Y, et al. Front Med (Lausanne). 2024 Nov 6;11:1457469. doi: 10.3389/fmed.2024.1457469. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39568743 Free PMC article. - Minimizing blood sampling in preterm infants.
Nissimov S, Sibrecht G, Weerasekara I, Bartocci M, Bruschettini M. Nissimov S, et al. Cochrane Database Syst Rev. 2024 Nov 19;11(11):CD016077. doi: 10.1002/14651858.CD016077. Cochrane Database Syst Rev. 2024. PMID: 39560051 Free PMC article. - Bone-conduction Hearing Aids: A Scoping Review.
Manuelli M, Migliorelli A, Moretti C, Borin M, Malagutti N, Bianchini C, Pelucchi S, Stomeo F, Ciorba A. Manuelli M, et al. Indian J Otolaryngol Head Neck Surg. 2024 Dec;76(6):5071-5079. doi: 10.1007/s12070-024-05042-7. Epub 2024 Sep 11. Indian J Otolaryngol Head Neck Surg. 2024. PMID: 39559027 - The value of serum Mac-2 binding protein glycosylation isomer in the diagnosis of liver fibrosis: a systematic review and meta-analysis.
Liu X, Zhang W, Ma B, Lv C, Sun M, Shang Q. Liu X, et al. Front Physiol. 2024 Oct 30;15:1382293. doi: 10.3389/fphys.2024.1382293. eCollection 2024. Front Physiol. 2024. PMID: 39558944 Free PMC article.
References
- Canadian Institutes of Health Research (2006) Randomized controlled trials registration/application checklist (12/2006). Available: http://www.cihr-irsc.gc.ca/e/documents/rct_reg_e.pdf. Accessed 26 May 2009.
- Young C, Horton R. Putting clinical trials into context. Lancet 2005;366:107-108. - PubMed
- Hemels ME, Vicente C, Sadri H, Masson MJ, Einarson TR. Quality assessment of meta-analyses of RCTs of pharmacotherapy in major depressive disorder. Curr Med Res Opin 2004;20:477-484. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources