Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach - PubMed (original) (raw)
Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach
Gerlinda E Hermann et al. J Neurosci. 2009.
Abstract
Bleeding head injury is associated with gastric stasis, a symptom of collapse of autonomic control of the gut described by Cushing around 1932. Recent work suggests that the proteinase thrombin, produced secondary to bleeding, may be the root cause. Results from our in vivo physiological studies show that fourth ventricular injection of PAR1 agonists, as well as thrombin itself, produced significant reductions in gastric transit in the awake rat. We expected that the PAR1 effect to inhibit gastric transit was the result of direct action on vagovagal reflex circuitry in the dorsal medulla. Surprisingly, our immunohistochemical studies demonstrated that PAR1 receptors are localized exclusively to the astrocytes and not the neurons in the nucleus of the solitary tract (NST; principal locus integrating visceral afferent input and part of the gastric vagovagal reflex control circuitry). Our in vitro calcium imaging studies of hindbrain slices revealed that PAR1 activation initially causes a dramatic increase in astrocytic calcium, followed seconds later by an increase in calcium signal in NST neurons. The neuronal effect, but not the astrocytic effect, of PAR1 activation was eliminated by glutamate receptor antagonism. TTX did not eliminate the effects of PAR1 activation on either glia or neurons. Thus, we propose that glia are the primary CNS sensors for PAR agonists and that the response of these glial cells drives the activity of adjacent (e.g., NST) neurons. These results show, for the first time, that changes in autonomic control can be directly signaled by glial detection of local chemical stimuli.
Figures
Figure 1.
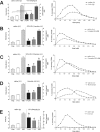
C13-octanoate gastric transit. Gastric emptying rate in an awake, freely moving animal can be monitored by determining the rate of appearance of 13C in respired CO2 that had been ingested as 13C-tagged sodium octanoate-doped meal. 13C in respired CO2 is indicative of the transit of the carbohydrate meal from the stomach to the duodenum. This 13C method captures the entire time course of the transit event (samples taken at 15 min intervals) and is displayed in the right-hand graphs. Specific parameters of this transit can be extracted for comparisons: _T_lag corresponds with the time at which rate of excretion of 13CO2 is maximal, _T_1/2 is the gastric half-emptying time (time when half of the label that is to be excreted has been excreted) and the GEC (a global index of rate of emptying). Each animal served as its own control. That is, the first session the animal receives the control injection (saline either i.p. or 4V, as appropriate); the next session the animal receives the test injection. Therefore, paired t test comparisons could be made between the saline control and the agonist condition for each animal. Examples of such paired gastric transit experiments of individual animals are seen in the right-hand graphs. In the left-hand column are group averages for the gastric transit parameters under each condition; statistical comparisons were only made within each condition group. Microinjection of thrombin (4 U) or the PAR1-selective agonist SFLLRN-NH2 (1 or 10 nmol) into the fourth ventricle caused a significant reduction in gastric transit as measured by _T_lag and _T_1/2 (B–D). Thrombin and 10 nmol SFLLRN-NH2 also slowed the overall GEC compared with their respective saline controls (C, D). For the sake of comparison, the dynamics of gastric transit suppression caused by intraperitoneal injection of either CCK-8 or LiCl are shown in A and E, respectively. *p < 0.05.
Figure 2.
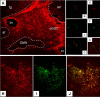
IHC identification of location of PAR1-positive cells in the DVC. A, Low-power view of the distribution of GFAP-ir astrocytes in the dorsal vagal complex. Note that the mNST contains substantially more astrocytes than surrounding areas, including the DMN and the ST. AP, Area postrema; bv, blood vessel; cc, central canal; fg, fasciculus gracilis. B–D, Single astrocytic fiber (GFAP-ir, red) in DMN possesses PAR1 receptors (PAR1-ir, green) as seen in merged image (D). The DMN is practically devoid of astrocytic profiles, though the few that are there show colocalization with PAR1-ir. E–G, Single GFAP profile within the fascicles of the ST; also possesses PAR1 receptors as seen in the merged image (G). This area shows the same pattern as seen in the DMN. H–J, Dense astrocytic label (GFAP-ir) in the mNST is extensively double labeled for PAR1-ir. Scale bars: A, 200 μm; B–D, 20 μm; E–G, 15 μm; H–J, 30 μm.
Figure 3.
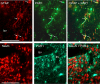
IHC identification of PAR1-positive cell types in NST. Double IHC studies demonstrated that PAR-ir (middle panels) is colocalized on astrocytes (GFAP-ir; top panels) but not on neurons (NeuN-ir; bottom panels) in the NST. Examples of colocalization are shown at arrows. bv, Blood vessels. Scale bars: top, 20 μm; bottom, 100 μm.
Figure 4.
Live cell imaging identification of neuronal versus glial characteristics. A, Hindbrain astrocytes (outlined in white) tend to be smaller, more irregular in shape, and have higher resting calcium levels relative to neurons (outlined in yellow). B, Response of individual neuron or astrocyte to 30 s exposure to 200 μ
m
glutamate.
Figure 5.
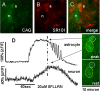
Response patterns of astrocytes and neurons to SFLLRN-NH2. A, CAG, the Ca2+ reporter dye, is taken up by both astrocytes (a) and neuronal cells (n). B, SR101 astrocyte-specific vital dye staining. C, Same field as both A and B; demonstration of in vitro glial and neuronal identification. A–C double fluorescence confocal images were taken through 509 and 607 nm bandpass filters. D, Responses of the same identified glial and neuron cells in A–C following 60 s bath exposure to 20 μ
m
SFLLRN-NH2. Note delay in onset and peak of neuronal response relative to glial response. Raw 500 nm long-pass filtered image frames on the right illustrate relative calcium levels in the astrocyte and neuron (outlined in dotted circles) at the approximately the peak of the SFLLRN-NH2 response (indicated by dashed vertical line through the response curves) and at rest.
Figure 6.
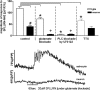
Response patterns of astrocytes and neurons to SFLLRN-NH2 during blockade. Glia and neurons have different response patterns (e.g., time of onset and magnitude) to SFLLRN-NH2. Magnitude of response to SFLLRN-NH2 is nearly three times greater in glia than neurons (left panel; # unpaired t test, p < 0.001).These differences are further delineated in the presence of a glutamate antagonist mixture (kynurenate, MK-801, plus AP3) which abolishes the neuronal “response” to SFLLRN-NH2, while the glial response appears only slightly muted (bar graph and raw data trace). When pretreated with the PLC inhibitor, U73122, for 10 min before the SFLLRN-NH2 challenge, PLC inhibition abolished the SFLLRN-NH2 response in both glia and, in turn, neurons. Last, when SFLLRN-NH2 is applied in the presence of TTX, the fundamental response to SFLLRN-NH2 in both glia and neurons is still seen. Unlike the glutamate blockade condition, under which the neuronal response was essentially abolished, under TTX conditions (which should be blocking action potential-dependent transmitter release), the neuronal response is not affected. These results provide evidence that the glia are the primary targets of SFLLRN-NH2, that neurons are subsequently activated by glutamate release from activated glia, and that glia may be under tonic vagal afferent influence. *ANOVA glial response, Dunnett's posttest p < 0.001; + ANOVA neuronal response, Dunnett's posttest p < 0.001.
Figure 7.
Schematic representation of possible relationship between afferents, astrocytes, and NST neurons. The results from these experiments suggest the hypothesis that local and circulating agents may act directly on glia to powerfully regulate the synaptic strength of hindbrain autonomic reflexes and visceral afferent circuits such as those involved in feeding behavior. Additionally, it is hypothesized that vagal afferent input can modulate the sensitivity of glial chemosensation via glutamatergic input. Such an intertwined system would allow for maximal range of feedback and feedforward regulation of reflexes.
Similar articles
- Thrombin action on astrocytes in the hindbrain of the rat disrupts glycemic and respiratory control.
Rogers RC, Hasser EM, Hermann GE. Rogers RC, et al. Am J Physiol Regul Integr Comp Physiol. 2020 Jun 1;318(6):R1068-R1077. doi: 10.1152/ajpregu.00033.2020. Epub 2020 Apr 22. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32320636 Free PMC article. - PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors.
Vance KM, Rogers RC, Hermann GE. Vance KM, et al. J Neurosci. 2015 Jan 14;35(2):776-85. doi: 10.1523/JNEUROSCI.3105-14.2015. J Neurosci. 2015. PMID: 25589770 Free PMC article. - Activation of astrocytic PAR1 receptors in the rat nucleus of the solitary tract regulates breathing through modulation of presynaptic TRPV1.
Huda R, Chang Z, Do J, McCrimmon DR, Martina M. Huda R, et al. J Physiol. 2018 Feb 1;596(3):497-513. doi: 10.1113/JP275127. Epub 2018 Jan 15. J Physiol. 2018. PMID: 29235097 Free PMC article. - Neurocoagulation from a Mechanistic Point of View in the Central Nervous System.
Shavit-Stein E, Berkowitz S, Gofrit SG, Altman K, Weinberg N, Maggio N. Shavit-Stein E, et al. Semin Thromb Hemost. 2022 Apr;48(3):277-287. doi: 10.1055/s-0041-1741569. Epub 2022 Jan 20. Semin Thromb Hemost. 2022. PMID: 35052009 Review. - Hindbrain astrocytes and glucose counter-regulation.
Rogers RC, Hermann GE. Rogers RC, et al. Physiol Behav. 2019 May 15;204:140-150. doi: 10.1016/j.physbeh.2019.02.025. Epub 2019 Feb 21. Physiol Behav. 2019. PMID: 30797812 Free PMC article. Review.
Cited by
- Thrombin action on astrocytes in the hindbrain of the rat disrupts glycemic and respiratory control.
Rogers RC, Hasser EM, Hermann GE. Rogers RC, et al. Am J Physiol Regul Integr Comp Physiol. 2020 Jun 1;318(6):R1068-R1077. doi: 10.1152/ajpregu.00033.2020. Epub 2020 Apr 22. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32320636 Free PMC article. - Glial cells modulate the synaptic transmission of NTS neurons sending projections to ventral medulla of Wistar rats.
Accorsi-Mendonça D, Zoccal DB, Bonagamba LG, Machado BH. Accorsi-Mendonça D, et al. Physiol Rep. 2013 Sep;1(4):e00080. doi: 10.1002/phy2.80. Epub 2013 Sep 17. Physiol Rep. 2013. PMID: 24303152 Free PMC article. - Glutamate receptor ion channels: structure, regulation, and function.
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Traynelis SF, et al. Pharmacol Rev. 2010 Sep;62(3):405-96. doi: 10.1124/pr.109.002451. Pharmacol Rev. 2010. PMID: 20716669 Free PMC article. Review. - Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance.
Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC, Hayes MR. Reiner DJ, et al. J Neurosci. 2016 Mar 23;36(12):3531-40. doi: 10.1523/JNEUROSCI.3579-15.2016. J Neurosci. 2016. PMID: 27013681 Free PMC article. - Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study.
Rogers RC, McDougal DH, Hermann GE. Rogers RC, et al. Brain Res. 2011 Apr 18;1385:47-55. doi: 10.1016/j.brainres.2011.02.029. Epub 2011 Feb 18. Brain Res. 2011. PMID: 21334313 Free PMC article.
References
- Akindipe OA, Faul JL, Vierra MA, Triadafilopoulos G, Theodore J. The surgical management of severe gastroparesis in heart/lung transplant recipients. Chest. 2000;117:907–910. - PubMed
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
- Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602–1607. - PubMed
- Coil JD, Rogers RC, Garcia J, Novin D. Conditioned taste aversions: vagal and circulatory mediation of the toxic unconditioned stimulus. Behav Biol. 1978;24:509–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK056373/DK/NIDDK NIH HHS/United States
- R21 HD047643-02/HD/NICHD NIH HHS/United States
- HD47643/HD/NICHD NIH HHS/United States
- R01 DK056373-09/DK/NIDDK NIH HHS/United States
- R01 NS060664-01A1/NS/NINDS NIH HHS/United States
- R21 HD047643-01A1/HD/NICHD NIH HHS/United States
- R01 DK056373-10/DK/NIDDK NIH HHS/United States
- R21 HD047643/HD/NICHD NIH HHS/United States
- R01 DK056373-08/DK/NIDDK NIH HHS/United States
- DK56373/DK/NIDDK NIH HHS/United States
- R01 DK056373-11/DK/NIDDK NIH HHS/United States
- R01 NS060664-02/NS/NINDS NIH HHS/United States
- NS60664/NS/NINDS NIH HHS/United States
- R01 NS060664/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources