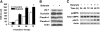Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers - PubMed (original) (raw)
Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers
Luying Peng et al. J Nutr. 2009 Sep.
Abstract
Butyrate, one of the SCFA, promotes the development of the intestinal barrier. However, the molecular mechanisms underlying the butyrate regulation of the intestinal barrier are unknown. To test the hypothesis that the effect of butyrate on the intestinal barrier is mediated by the regulation of the assembly of tight junctions involving the activation of the AMP-activated protein kinase (AMPK), we determined the effect of butyrate on the intestinal barrier by measuring the transepithelial electrical resistance (TER) and inulin permeability in a Caco-2 cell monolayer model. We further used a calcium switch assay to study the assembly of epithelial tight junctions and determined the effect of butyrate on the assembly of epithelial tight junctions and AMPK activity. We demonstrated that the butyrate treatment increased AMPK activity and accelerated the assembly of tight junctions as shown by the reorganization of tight junction proteins, as well as the development of TER. AMPK activity was also upregulated by butyrate during calcium switch-induced tight junction assembly. Compound C, a specific AMPK inhibitor, inhibited the butyrate-induced activation of AMPK. The facilitating effect of butyrate on the increases in TER in standard culture media, as well as after calcium switch, was abolished by compound C. We conclude that butyrate enhances the intestinal barrier by regulating the assembly of tight junctions. This dynamic process is mediated by the activation of AMPK. These results suggest an intriguing link between SCFA and the intracellular energy sensor for the development of the intestinal barrier.
Figures
FIGURE 1
Effects of butyrate on barrier function, expressions of tight junction proteins, and AMPK phosphorylation in Caco-2 cell monolayers. (A) Cells were grown in the trans-well inserts until confluence and then incubated with or without 2 mmol/L of butyrate for 72 h. Values are means + SE, n = 6. *Different from control at that time, P < 0.05. (B) After 72 h treatment with medium alone or with 2 mmol/L of butyrate, cell lysates were immunoblotted with the specific antibodies indicated. (C) Total cell lysates from the indicated time points after treatment with medium alone or with 2 mmol/L of butyrate were subjected to immunoblotting for pAMPK, total AMPK, pACC, and actin with specific antibodies.
FIGURE 2
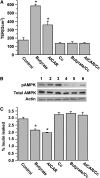
AMPK is involved in butyrate regulation of the barrier function in Caco-2 cell monolayers. (A) Caco-2 cells were incubated for 72 h in trans-well inserts with 2 mmol/L butyrate, 0.5 mmol/L AICAR, 10 _μ_mol/L compound C (Cc), or butyrate with Cc and AICAR with Cc, respectively. (B) Extracts from Caco-2 cells at 72 h were immunoblotted for pAMPK, total AMPK, and actin. Lanes 1–6 are medium control, butyrate at 2 mmol/L, AICAR at 0.5 mmol/L, Cc at 10 _μ_mol/L, butyrate+Cc, and AICAR+Cc, respectively. (C) Paracellular flux was determined using FITC-inulin probes in Caco-2 cell monolayers treated similarly for 72 h. In A and C, values are means + SE, n = 6. *Different from control, P < 0.05.
FIGURE 3
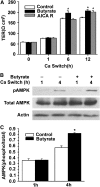
Effects of butyrate on the tight junction assembly and AMPK activation in Caco-2 cell monolayers after calcium switch. (A) After incubation in a low-calcium medium for 16 h, cell monolayers were switched to a regular calcium medium alone or with 2 mmol/L of butyrate, or with 0.5 mmol/L of AICAR. Values are means + SE, n = 5. *Different from control at that time, P ≤ 0.05. B. Total cell lysates from untreated cells or those treated with 2 mmol/L of butyrate at indicated calcium-switch time points were subjected to immunoblotting for pAMPK, total AMPK, and actin with specific antibodies. (C) AMPK activity was expressed as the ratio of the phosphorylated form of the protein to total protein. Values are means + SE, n = 8. *Different from control at that time, P ≤ 0.05.
FIGURE 4
Butyrate facilitates redistribution of ZO-1 and occludin during calcium switch-induced tight junction assembly Caco-2 cells were grown in normal DMEM for 2–3 d to confluence and subjected to incubation with a low-calcium medium for 16 h. When calcium was switched back into the medium in the absence or presence of butyrate at 2 mmol/L for indicated times, the cell monolayers were fixed and the distribution of ZO-1 (A) and occludin (B) in intercellular junctions was visualized by probing the target proteins with specific antibodies. The fluorescence images were obtained with a confocal laser scanning microscope (scale bars = 20 _μ_m).
FIGURE 5
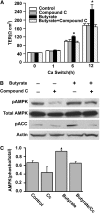
Specific AMPK inhibitor abolishes the facilitating effect of butyrate on tight junction assembly. After 16 h of incubation with low-calcium medium, Caco-2 cells were treated with normal medium/vehicle (medium), 10 _μ_mol/L of compound C, 2 mmol/L of butyrate, and 2 mmol/L of butyrate plus 10 _μ_mol/L of compound C, respectively. (A) The facilitating effect of butyrate on the increases in TER after calcium switch was abolished by the co-treatment of compound C. Values are means + SE, n = 4. *Different from other groups at that time, P < 0.05. (B) Total lysates were isolated from the cells after 4 h of treatment and subjected to immunoblotting for pAMPK, total AMPK, pACC, and actin with specific antibodies. Compound C significantly inhibited the butyrate-induced activation of AMPK. (C) Active AMPK is defined as the band intensity of pAMPK Thr-172 to that of total AMPK. Values are means + SE, n = 8. *Different from control, P < 0.05.
Similar articles
- AMPK Activation Promotes Tight Junction Assembly in Intestinal Epithelial Caco-2 Cells.
Olivier S, Leclerc J, Grenier A, Foretz M, Tamburini J, Viollet B. Olivier S, et al. Int J Mol Sci. 2019 Oct 18;20(20):5171. doi: 10.3390/ijms20205171. Int J Mol Sci. 2019. PMID: 31635305 Free PMC article. - Inhibition of p38 mitogen-activated protein kinase attenuates butyrate-induced intestinal barrier impairment in a Caco-2 cell monolayer model.
Huang XZ, Li ZR, Zhu LB, Huang HY, Hou LL, Lin J. Huang XZ, et al. J Pediatr Gastroenterol Nutr. 2014 Aug;59(2):264-9. doi: 10.1097/MPG.0000000000000369. J Pediatr Gastroenterol Nutr. 2014. PMID: 24625969 - Microbial metabolite n-butyrate upregulates intestinal claudin-23 expression through SP1 and AMPK pathways in mouse colon and human intestinal Caco-2 cells.
Xu W, Ishii Y, Rini DM, Yamamoto Y, Suzuki T. Xu W, et al. Life Sci. 2023 Sep 15;329:121952. doi: 10.1016/j.lfs.2023.121952. Epub 2023 Jul 17. Life Sci. 2023. PMID: 37467886 - AMPK in regulation of apical junctions and barrier function of intestinal epithelium.
Zhu MJ, Sun X, Du M. Zhu MJ, et al. Tissue Barriers. 2018;6(2):1-13. doi: 10.1080/21688370.2018.1487249. Epub 2018 Aug 21. Tissue Barriers. 2018. PMID: 30130441 Free PMC article. Review. - Implications of AMPK in the Formation of Epithelial Tight Junctions.
Rowart P, Wu J, Caplan MJ, Jouret F. Rowart P, et al. Int J Mol Sci. 2018 Jul 13;19(7):2040. doi: 10.3390/ijms19072040. Int J Mol Sci. 2018. PMID: 30011834 Free PMC article. Review.
Cited by
- Elevated levels of butyric acid in the jejunum of an animal model of broiler chickens: from early onset of Clostridium perfringens infection to clinical disease of necrotic enteritis.
Gautam H, Shaik NA, Banaganapalli B, Popowich S, Subhasinghe I, Ayalew LE, Mandal R, Wishart DS, Tikoo S, Gomis S. Gautam H, et al. J Anim Sci Biotechnol. 2024 Nov 2;15(1):144. doi: 10.1186/s40104-024-01105-5. J Anim Sci Biotechnol. 2024. PMID: 39487547 Free PMC article. - The Gut Microbiome Is Altered in Postmenopausal Women With Osteoporosis and Osteopenia.
Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, Coad J, Kruger MC. Rettedal EA, et al. JBMR Plus. 2021 Jan 19;5(3):e10452. doi: 10.1002/jbm4.10452. eCollection 2021 Mar. JBMR Plus. 2021. PMID: 33778322 Free PMC article. - Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation.
Bavaro AR, Di Biase M, Linsalata V, D'Antuono I, Di Stefano V, Lonigro SL, Garbetta A, Valerio F, Melilli MG, Cardinali A. Bavaro AR, et al. Foods. 2024 Jun 8;13(12):1815. doi: 10.3390/foods13121815. Foods. 2024. PMID: 38928756 Free PMC article. - Claudin-3 and occludin tissue content in the glands of colonic mucosa with and without a fecal stream.
Martinez CA, de Campos FG, de Carvalho VR, de Castro Ferreira C, Rodrigues MR, Sato DT, Pereira JA. Martinez CA, et al. J Mol Histol. 2015 Apr;46(2):183-94. doi: 10.1007/s10735-015-9610-y. Epub 2015 Feb 4. J Mol Histol. 2015. PMID: 25649016 - Colonic Microbiota Improves Fiber Digestion Ability and Enhances Absorption of Short-Chain Fatty Acids in Local Pigs of Hainan.
Xue P, Xue M, Luo Y, Tang Q, Wang F, Sun R, Song Y, Chao Z, Fang M. Xue P, et al. Microorganisms. 2024 May 21;12(6):1033. doi: 10.3390/microorganisms12061033. Microorganisms. 2024. PMID: 38930415 Free PMC article.
References
- Walker WA. Development of the intestinal mucosal barrier. J Pediatr Gastroenterol Nutr. 2002;34:S33–9. - PubMed
- Rouwet EV, Heineman E, Buurman WA, Riet GT, Ramsay G, Blanco CE. Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res. 2002;51:64–70. - PubMed
- Udall JN, Pang K, Fritze L, Kleinman R, Walker WA. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr Res. 1981;15:241–4. - PubMed
- Udall JN, Colony P, Fritze L, Pang K, Trier JS, Walker WA. Development of gastrointestinal mucosal barrier. II. The effect of natural versus artificial feeding on intestinal permeability to macromolecules. Pediatr Res. 1981;15:245–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources