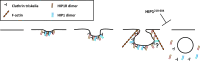HIP1 exhibits an early recruitment and a late stage function in the maturation of coated pits - PubMed (original) (raw)
HIP1 exhibits an early recruitment and a late stage function in the maturation of coated pits
Irit Gottfried et al. Cell Mol Life Sci. 2009 Sep.
Abstract
Huntingtin interacting protein 1 (HIP1) is an accessory protein of the clathrin-mediated endocytosis (CME) pathway, yet its precise role and the step at which it becomes involved are unclear. We employed live-cell imaging techniques to focus on the early steps of CME and characterize HIP1 dynamics. We show that HIP1 is highly colocalized with clathrin at the plasma membrane and shares similar dynamics with a subpopulation of clathrin assemblies. Employing transferrin receptor fused to pHluorin, we distinguished between open pits to which HIP1 localizes and newly internalized vesicles that are devoid of HIP1. Moreover, shRNA knockdown of clathrin compromised HIP1 membranal localization, unlike the reported behavior of Sla2p. HIP1 fragment, lacking its ANTH and Talin-like domains, inhibits internalization of transferrin, but retains colocalization with membranal clathrin assemblies. These data demonstrate HIP1's role in pits maturation and formation of the coated vesicle, and its strong dependence on clathrin for membranal localization.
Figures
Fig. 1
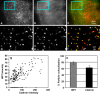
HIP1 is colocalized with clathrin at the PM. TIRF images of a cell coexpressing tdTomato-CLC (a) and EGFP-HIP1 (b). c Merged image (clathrin in red, HIP1 in green). d–f Enlarged and filtered images of the regions marked in a–c, respectively. Note that most assemblies are colocalized, though some HIP1-only (arrowhead) or clathrin-only (arrow) spots can be found. g Scatter plot showing the correlation between HIP1 and clathrin assemblies intensity (n = 171 and 212, respectively). h Averaged assemblies colocalization of HIP1 and clathrin shows strong colocalization with clathrin at the PM (mean ± SEM, regions from five cells)
Fig. 2
HIP1 and clathrin display similar dynamics at the PM. a An example of a short-lived (18 s) HIP1 assembly (box). b Histograms displaying the lifetime distributions for the two proteins. Curves represent log-normal fittings for the data. Hatched curves represent the fit for the predicted shorter lived subpopulation of clathrin assemblies (CSP) (n = 661 and 439; from 10 and 11 cells for clathrin and HIP1, respectively). c Histograms displaying the point-to-point velocity distribution for the two proteins. Curves represent log-normal fittings for the data. Hatched curves represent the fit for the predicted faster-moving subpopulation of clathrin assemblies (CSP). d Correlation plot displaying assemblies velocity vs. lifetime. Note clathrin’s subpopulation of assemblies (CSP) with shorter lifetimes and greater mobility
Fig. 3
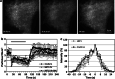
Application of 1-butanol dissociates HIP1 from the PM. a Example of changes in TIRF images of HIP1 following 2% butanol application and washout. b Quantification of the changes in assemblies number in response to butanol treatment (clathrin: n = 5; HIP1: n = 7; HIP1218–604: n = 5). Gray bar represents time of butanol application. (Error bars presented for 1/3 of the points.) c Averaged changes in normalized intensity of individual HIP1 and clathrin assemblies that appeared after butanol washout. Minimal intensity for detection was usually about 25%. t = 0 represents maximal intensity (n = 10 pairs)
Fig. 4
Hypertonic sucrose solution increases the density of HIP1 and clathrin assemblies. a Filtered TIRF images of a cell coexpressing tdTomato-CLC and EGFP-HIP1, before (upper panel) and after (lower panel) sucrose treatment. Merged images show colocalized pixels in white and others in their relevant color (clathrin in red, HIP1 in green). b Changes in assembly density following sucrose application (mean ± SEM, n = 5, P < 0.005, paired _t_-test)
Fig. 5
Clathrin silencing reduces the number of HIP1’s assemblies at the PM. a TIRF images of HeLa cells expressing either HIP1-GFP (FL4days) alone, or in combination with scramble shRNA (SCRsh) or clathrin shRNA (CHCsh). b Assembly density is displayed for the three groups (mean ± SEM, regions from 13, 14 and 18 cells, respectively, P < 0.0001, one-way ANOVA). c Fluorescence changes were scored as an indication of vesicle activity (appearance, disappearance and flickering) (mean ± SEM, regions from 37, 36 and 41 cells, respectively, P < 0.0001, Kruskal-Wallis test; difference between FL4days and SCRsh was not significant according to Dunn’s comparison test)
Fig. 6
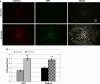
HIP1 colocalizes with transferrin receptor (TfR). a A region of a cell coexpressing tdTomato-HIP1 (red) and TfR-pHl (green) was visualized in TIRF, under two pH conditions (left, pH 7.4; right, pH 5.5). Both images are filtered under the same conditions, but scaled differently, so that the low-intensity internalized TfR-pHl vesicles would be visible. b Colocalization of HIP1 and TfR assemblies was measured under the two pH conditions (mean ± SEM, regions from seven cells, P < 0.005, paired _t_-test)
Fig. 7
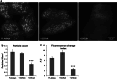
Expression of HIP1218–604 impairs transferrin uptake, but does not affect the appearance of membranal transferrin receptor assemblies. a COS7 cells expressing either GFP, HIP1-GFP or HIP1218–604-GFP were incubated with 15 μg/ml transferrin-alexa546 for 5 min. Cells expressing the proteins are outlined in the corresponding transferrin uptake image. b Quantification of transferrin accumulation in cells described in a. Bars represent mean ± SEM. Number of cells analyzed: 102, 82 and 74 for GFP, HIP1 and HIP1218–604, respectively, from two independent experiments. P < 0.001. c Transferrin receptor assemblies were counted in TIRF images of cells expressing the TfR-pHlu alone or in combination with HIP1 or HIP1218–604 and found to be similar in these conditions. Bars represent mean ± SEM. Three experiments, total number of cells analyzed 15, 23 and 20 for GFP, HIP1 and HIP1218–604, respectively
Fig. 8
A schematic representation of HIP1’s involvement in the initial steps of clathrin-mediated endocytosis. HIP1 dimers are recruited to coated pits, parallel to clathrin and HIP1R [22] arrival. HIP1’s actin-binding capabilities are controversial, therefore marked by a question mark in the scheme. HIP1 dissociates from the coated pit together with clathrin, and it is hardly found on coated vesicles. Additionally, HIP1218–604 blocks endocytosis following coated pit formation, suggesting that HIP1 functions in the last steps of coated pit maturation and the formation of coated vesicles
Similar articles
- An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles.
Engqvist-Goldstein AE, Kessels MM, Chopra VS, Hayden MR, Drubin DG. Engqvist-Goldstein AE, et al. J Cell Biol. 1999 Dec 27;147(7):1503-18. doi: 10.1083/jcb.147.7.1503. J Cell Biol. 1999. PMID: 10613908 Free PMC article. - The huntingtin interacting protein HIP1 is a clathrin and alpha-adaptin-binding protein involved in receptor-mediated endocytosis.
Waelter S, Scherzinger E, Hasenbank R, Nordhoff E, Lurz R, Goehler H, Gauss C, Sathasivam K, Bates GP, Lehrach H, Wanker EE. Waelter S, et al. Hum Mol Genet. 2001 Aug 15;10(17):1807-17. doi: 10.1093/hmg/10.17.1807. Hum Mol Genet. 2001. PMID: 11532990 - TTP specifically regulates the internalization of the transferrin receptor.
Tosoni D, Puri C, Confalonieri S, Salcini AE, De Camilli P, Tacchetti C, Di Fiore PP. Tosoni D, et al. Cell. 2005 Dec 2;123(5):875-88. doi: 10.1016/j.cell.2005.10.021. Cell. 2005. PMID: 16325581 - The Sla2p/HIP1/HIP1R family: similar structure, similar function in endocytosis?
Gottfried I, Ehrlich M, Ashery U. Gottfried I, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):187-91. doi: 10.1042/BST0380187. Biochem Soc Trans. 2010. PMID: 20074057 Review. - Evolving models for assembling and shaping clathrin-coated pits.
Chen Z, Schmid SL. Chen Z, et al. J Cell Biol. 2020 Sep 7;219(9):e202005126. doi: 10.1083/jcb.202005126. J Cell Biol. 2020. PMID: 32770195 Free PMC article. Review.
Cited by
- Endocytic pathways involved in filovirus entry: advances, implications and future directions.
Bhattacharyya S, Mulherkar N, Chandran K. Bhattacharyya S, et al. Viruses. 2012 Dec;4(12):3647-64. doi: 10.3390/v4123647. Viruses. 2012. PMID: 23342373 Free PMC article. Review. - The first five seconds in the life of a clathrin-coated pit.
Cocucci E, Aguet F, Boulant S, Kirchhausen T. Cocucci E, et al. Cell. 2012 Aug 3;150(3):495-507. doi: 10.1016/j.cell.2012.05.047. Cell. 2012. PMID: 22863004 Free PMC article. - Quantitative analysis of endocytosis with cytoplasmic pHluorin chimeras.
Prosser DC, Whitworth K, Wendland B. Prosser DC, et al. Traffic. 2010 Sep;11(9):1141-50. doi: 10.1111/j.1600-0854.2010.01088.x. Epub 2010 Jun 15. Traffic. 2010. PMID: 20626707 Free PMC article. - Differential requirements for clathrin endocytic pathway components in cellular entry by Ebola and Marburg glycoprotein pseudovirions.
Bhattacharyya S, Hope TJ, Young JA. Bhattacharyya S, et al. Virology. 2011 Oct 10;419(1):1-9. doi: 10.1016/j.virol.2011.07.018. Epub 2011 Aug 19. Virology. 2011. PMID: 21855102 Free PMC article. - Functional characterization of 67 endocytic accessory proteins using multiparametric quantitative analysis of CCP dynamics.
Bhave M, Mino RE, Wang X, Lee J, Grossman HM, Lakoduk AM, Danuser G, Schmid SL, Mettlen M. Bhave M, et al. Proc Natl Acad Sci U S A. 2020 Dec 15;117(50):31591-31602. doi: 10.1073/pnas.2020346117. Epub 2020 Nov 30. Proc Natl Acad Sci U S A. 2020. PMID: 33257546 Free PMC article.
References
- Jones AL, Hradek GT, Hornick C, Renaud G, Windler EE, Havel RJ. Uptake and processing of remnants of chylomicrons and very low density lipoproteins by rat liver. J Lipid Res. 1984;25:1151–1158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials