Forced abstinence model of relapse to study pharmacological treatments of substance use disorder - PubMed (original) (raw)
Review
Forced abstinence model of relapse to study pharmacological treatments of substance use disorder
Carmela M Reichel et al. Curr Drug Abuse Rev. 2009 May.
Abstract
Understanding and preventing relapse to drug use is one of the most difficult challenges faced by clinicians and practitioners in the struggle to help people remain abstinent. In this paper, we review basic preclinical research on forced abstinence periods that identify the neural substrates involved and neural adaptations that occur after a drug-free period. Our attention focuses on forced abstinence after self-administration because of its promise for translational research in the development of candidate medications to reduce relapse. This model requires subjects (often rats) to initially acquire drug self-administration. However, rather than extinguishing behavior with daily drug-free sessions as in the reinstatement model of drug seeking, subjects are removed from the self-administration situation and do not receive any exposure to the drug. Notably, the integrity of the drug-taking behavior and the drug-associated cues in the drug-taking environment are preserved because they are not experienced in the absence of the drug. Research shows time dependent increases in drug-seeking following forced abstinence periods. More so, neural substrates and adaptations within the mesocorticolimbic system and the nigrostriatal system have been identified that contribute to increased drug seeking following abstinence. From a translational perspective, behavioral and pharmacological treatment of substance use disorder often starts during this initial abstinence period (either forced or voluntary). The forced abstinence model simulates some of the features of this treatment situation and thus allows for the study of potential treatments that alter relapse of drug-seeking behaviors along with the accompanying neurobiological changes.
Figures
Figure 1
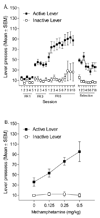
This figure shows active and inactive lever presses (Mean ± SEM) for rats (n= 14) trained to self-administer 0.05 mg/kg/infusion methamphetamine. Panel A depicts the acquisition of the lever press response on FR 1, 3, and 5 schedules of reinforcement and the extinction of such a response. Panel B illustrates drug-primed reinstatement tested with 0, 0.125, 0.25, and 0.5 mg/kg methamphetamine IP.
Figure 2
This figure shows active and inactive lever presses (Mean ± SEM) for rats (n=10) trained to self-administer 0.05 mg/kg/infusion methamphetamine. Following stable methamphetamine self-administration (last 7 days of drug access are shown), rats were given 14 days of forced abstinence followed by 2 consecutive relapse tests occurring 24 hr apart. These tests were conducted with contingent presentations of the stimulus complex on an FR5 schedule of reinforcement.
Figure 3
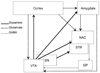
This figure is a schematic representation of the mesocorticolimbic and nigrostriatal systems that are important in relapse to cocaine seeking following abstinence. Abbreviations: NAC, nucleus accumbens; STR, striatum (caudate and putamen); GP, globus pallidus; SN, substantia nigra; VTA, ventral tegmental area.
Similar articles
- Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence.
Venniro M, Caprioli D, Shaham Y. Venniro M, et al. Prog Brain Res. 2016;224:25-52. doi: 10.1016/bs.pbr.2015.08.004. Epub 2015 Nov 4. Prog Brain Res. 2016. PMID: 26822352 Review. - Roflumilast treatment during forced abstinence reduces relapse to methamphetamine seeking and taking.
Baek JJ, Kline H, Deveau CM, Yamamoto BK. Baek JJ, et al. Addict Biol. 2022 Jan;27(1):e13082. doi: 10.1111/adb.13082. Epub 2021 Aug 7. Addict Biol. 2022. PMID: 34363284 Free PMC article. - Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model.
Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, Marchant NJ, Lucantonio F, Schoenbaum G, Bossert JM, Shaham Y. Caprioli D, et al. Biol Psychiatry. 2015 Oct 1;78(7):463-73. doi: 10.1016/j.biopsych.2015.02.018. Epub 2015 Feb 23. Biol Psychiatry. 2015. PMID: 25861699 Free PMC article. - Relapse after electric barrier-induced voluntary abstinence: A review.
Negishi K, Fredriksson I, Bossert JM, Zangen A, Shaham Y. Negishi K, et al. Curr Opin Neurobiol. 2024 Jun;86:102856. doi: 10.1016/j.conb.2024.102856. Epub 2024 Mar 19. Curr Opin Neurobiol. 2024. PMID: 38508102 Review. - Relapse to opioid seeking in rat models: behavior, pharmacology and circuits.
Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Reiner DJ, et al. Neuropsychopharmacology. 2019 Feb;44(3):465-477. doi: 10.1038/s41386-018-0234-2. Epub 2018 Oct 6. Neuropsychopharmacology. 2019. PMID: 30293087 Free PMC article. Review.
Cited by
- AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving.
Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, Dolubizno H, Stefanik MT, Murray CH, Sakas C, Wolf ME. Scheyer AF, et al. Biol Psychiatry. 2016 Nov 1;80(9):661-670. doi: 10.1016/j.biopsych.2016.04.003. Epub 2016 Apr 12. Biol Psychiatry. 2016. PMID: 27264310 Free PMC article. - Drug memory reconsolidation: from molecular mechanisms to the clinical context.
Milton AL. Milton AL. Transl Psychiatry. 2023 Dec 1;13(1):370. doi: 10.1038/s41398-023-02666-1. Transl Psychiatry. 2023. PMID: 38040677 Free PMC article. Review. - Combined action of MK-801 and ceftriaxone impairs the acquisition and reinstatement of morphine-induced conditioned place preference, and delays morphine extinction in rats.
Fan Y, Niu H, Rizak JD, Li L, Wang G, Xu L, Ren H, Lei H, Yu H. Fan Y, et al. Neurosci Bull. 2012 Oct;28(5):567-76. doi: 10.1007/s12264-012-1269-8. Epub 2012 Oct 3. Neurosci Bull. 2012. PMID: 23054634 Free PMC article. - The impact of cocaine and heroin drug history on motivation and cue sensitivity in a rat model of polydrug abuse.
Crummy EA, Donckels EA, Baskin BM, Bentzley BS, Ferguson SM. Crummy EA, et al. Psychopharmacology (Berl). 2020 Jan;237(1):55-68. doi: 10.1007/s00213-019-05349-2. Epub 2019 Aug 28. Psychopharmacology (Berl). 2020. PMID: 31463541 Free PMC article. - The co-use of nicotine and prescription psychostimulants: A review of their behavioral and neuropharmacological interactions.
McNealy KR, Weyrich L, Bevins RA. McNealy KR, et al. Drug Alcohol Depend. 2023 Jul 1;248:109906. doi: 10.1016/j.drugalcdep.2023.109906. Epub 2023 May 4. Drug Alcohol Depend. 2023. PMID: 37216808 Free PMC article. Review.
References
- Drugs, Brains, and Behavior: The Science of Addiction. National Institute of Health Publication Number 07-5605
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. The New England Journal of Medicine. 1996;334:965–972. - PubMed
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. - PubMed
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implication for treatment, insurance, and outcomes evaluation. JAMA. 284:1689–1695. - PubMed
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of relapse: histroy, methodology and major findings. Psychopharmacol. 2003;168:3–20. - PubMed
Publication types
MeSH terms
Grants and funding
- F31 DA023283/DA/NIDA NIH HHS/United States
- R01 DA018114/DA/NIDA NIH HHS/United States
- R01 DA018114-05/DA/NIDA NIH HHS/United States
- DA018114/DA/NIDA NIH HHS/United States
- DA023283/DA/NIDA NIH HHS/United States