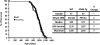Overexpression of Mn superoxide dismutase does not increase life span in mice - PubMed (original) (raw)
. 2009 Nov;64(11):1114-25.
doi: 10.1093/gerona/glp100. Epub 2009 Jul 24.
Viviana I Pérez, Wook Song, Michael S Lustgarten, Adam B Salmon, James Mele, Wenbo Qi, Yuhong Liu, Hanyu Liang, Asish Chaudhuri, Yuji Ikeno, Charles J Epstein, Holly Van Remmen, Arlan Richardson
Affiliations
- PMID: 19633237
- PMCID: PMC2759571
- DOI: 10.1093/gerona/glp100
Overexpression of Mn superoxide dismutase does not increase life span in mice
Youngmok C Jang et al. J Gerontol A Biol Sci Med Sci. 2009 Nov.
Abstract
Genetic manipulations of Mn superoxide dismutase (MnSOD), SOD2 expression have demonstrated that altering the level of MnSOD activity is critical for cellular function and life span in invertebrates. In mammals, Sod2 homozygous knockout mice die shortly after birth, and alterations of MnSOD levels are correlated with changes in oxidative damage and in the generation of mitochondrial reactive oxygen species. In this study, we directly tested the effects of overexpressing MnSOD in young (4-6 months) and old (26-28 months) mice on mitochondrial function, levels of oxidative damage or stress, life span, and end-of-life pathology. Our data show that an approximately twofold overexpression of MnSOD throughout life in mice resulted in decreased lipid peroxidation, increased resistance against paraquat-induced oxidative stress, and decreased age-related decline in mitochondrial ATP production. However, this change in MnSOD expression did not alter either life span or age-related pathology.
Figures
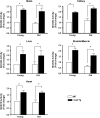
Figure 1.
MnSOD activity in various tissues of wild-type (WT) and Sod2 Tg mice. The activity of MnSOD was measured in tissue homogenates isolated from brain, kidney, liver, skeletal muscle, and heart of young and old WT (open bar) and Sod2 Tg (solid bar) mice, determined using native gels described in the Materials and Methods section. The data were obtained from four to six mice per group and expressed as mean ± SEM. The MnSOD activity for young WT mice was normalized to 1. Data were statistically analyzed using two-way analysis of variance with the Bonferroni test mice; an asterisk denotes those values that are significantly different from young WT mice at the p < .05 level.
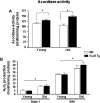
Figure 2.
Aconitase activity and H2O2 generation in skeletal muscle mitochondria from wild-type (WT) and Sod2 Tg mice. Aconitase activity (A) and H2O2 production (B) were determined in mitochondria isolated from skeletal muscle of young and old WT (open bars) and Sod2 Tg mice (solid bars). The data are the mean of four to six animals ± SEM and were analyzed by the nonparametric test of analysis of variance (with Bonferroni’s post hoc). The asterisk denotes a statistically significant difference between WT and Sod2 Tg mice (aconitase activity) and young and old mice (H2O2 production) at the p < .05 level.
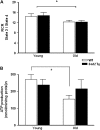
Figure 3.
Mitochondrial respiration and ATP production in skeletal muscle mitochondria from wild-type (WT) and Sod2 Tg mice. The mitochondrial respiration control ratio (A) and ATP production (B) were determined in mitochondria isolated from skeletal muscle of young and old WT (open bars) and Sod2 Tg (solid bars) mice using complex II-linked substrate. The data are the mean of six animals ± SEM and were analyzed by the nonparametric test of analysis of variance (with Bonferroni’s post hoc). The asterisk denotes a statistically significant difference between young and old mice at the p < .05 level.
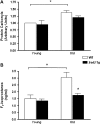
Figure 4.
Oxidative damage in skeletal muscle from young and old wild-type (WT) and Sod2 Tg mice. Protein carbonyl (A) and F2-isoprostane (B) levels were measured in skeletal muscle from young and old mice of Sod2 Tg (solid bars) and WT (open bars) mice. Protein carbonyl levels were determined as described by (42) and F2-isoprostanes determined as described by (43). Data shown are the mean ± SEM for six animals per group and were analyzed using analysis of variance with Bonferroni’s post hoc test. The asterisks denote a statistically significant difference at the p < .05 level.
Figure 5.
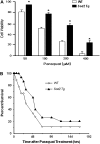
Effect of overexpressing MnSOD on sensitivity to oxidative stress. (A) Primary cultures of murine embryonic fibroblasts (MEFs) isolated from Sod2 Tg and wild-type (WT) mice were treated with various doses of paraquat for 48 hours. Cell viability was measured by the neutral red assay as described in the Materials and Methods section. All values represent the mean ± SEM from three different animals. The data were analyzed by a two-way analysis of variance with a follow-up Tukey’s multiple range test. The asterisk denotes those values that are significantly different at p < .05 between MEFs isolated from Sod2 Tg compared with WT mice. (B) Paraquat (50 mg/kg) was administered to 12 WT (open diamonds) and 13 Sod2 Tg (solid triangles) mice, and the survival of the mice was followed over 7 days. The survival curves were statistically analyzed using the log-rank test and shown to be significant at the p < .05 level.
Figure 6.
Life span of Sod2 Tg and WT mice. The survival curves of wild-type (WT; open symbols) and Sod2 Tg (solid symbols) mice were obtained from 50 male Sod2 Tg and 47 male WT mice. The survival data in the table are expressed in days. Mean survival (±SEM) for each group was compared with the WT group by performing a Student’s t test upon log-transformed survival times from the respective groups. The mean, median, 10%, and maximum survivals for each group were compared with the WT group using a score test adapted from Wang and colleagues (67).
Similar articles
- Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging.
Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Mansouri A, et al. Mech Ageing Dev. 2006 Mar;127(3):298-306. doi: 10.1016/j.mad.2005.11.004. Epub 2006 Jan 6. Mech Ageing Dev. 2006. PMID: 16405961 - Role of mitochondrial superoxide dismutase in contraction-induced generation of reactive oxygen species in skeletal muscle extracellular space.
McArdle A, van der Meulen J, Close GL, Pattwell D, Van Remmen H, Huang TT, Richardson AG, Epstein CJ, Faulkner JA, Jackson MJ. McArdle A, et al. Am J Physiol Cell Physiol. 2004 May;286(5):C1152-8. doi: 10.1152/ajpcell.00322.2003. Epub 2004 Jan 14. Am J Physiol Cell Physiol. 2004. PMID: 15075214 - Manganese superoxide dismutase deficiency exacerbates the mitochondrial ROS production and oxidative damage in Chagas disease.
Wen JJ, Garg NJ. Wen JJ, et al. PLoS Negl Trop Dis. 2018 Jul 25;12(7):e0006687. doi: 10.1371/journal.pntd.0006687. eCollection 2018 Jul. PLoS Negl Trop Dis. 2018. PMID: 30044789 Free PMC article. - Invited review: manganese superoxide dismutase in disease.
Macmillan-Crow LA, Cruthirds DL. Macmillan-Crow LA, et al. Free Radic Res. 2001 Apr;34(4):325-36. doi: 10.1080/10715760100300281. Free Radic Res. 2001. PMID: 11328670 Review. - The use of transgenic and mutant mice to study oxygen free radical metabolism.
Huang TT, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. Huang TT, et al. Ann N Y Acad Sci. 1999;893:95-112. doi: 10.1111/j.1749-6632.1999.tb07820.x. Ann N Y Acad Sci. 1999. PMID: 10672232 Review.
Cited by
- Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6.
Tyrrell DJ, Goldstein DR. Tyrrell DJ, et al. Nat Rev Cardiol. 2021 Jan;18(1):58-68. doi: 10.1038/s41569-020-0431-7. Epub 2020 Sep 11. Nat Rev Cardiol. 2021. PMID: 32918047 Free PMC article. Review. - The use of the Cre/loxP system to study oxidative stress in tissue-specific manganese superoxide dismutase knockout models.
Marecki JC, Parajuli N, Crow JP, MacMillan-Crow LA. Marecki JC, et al. Antioxid Redox Signal. 2014 Apr 1;20(10):1655-70. doi: 10.1089/ars.2013.5293. Epub 2013 Jun 20. Antioxid Redox Signal. 2014. PMID: 23641945 Free PMC article. Review. - Strategies for reducing or preventing the generation of oxidative stress.
Poljsak B. Poljsak B. Oxid Med Cell Longev. 2011;2011:194586. doi: 10.1155/2011/194586. Epub 2011 Dec 10. Oxid Med Cell Longev. 2011. PMID: 22191011 Free PMC article. Review. - Transcriptional repression of mitochondrial function in aging: a novel role for the silencing mediator of retinoid and thyroid hormone receptors co-repressor.
Liu S, Reilly SM, Lee CH. Liu S, et al. Antioxid Redox Signal. 2013 Jul 20;19(3):299-309. doi: 10.1089/ars.2011.4413. Epub 2012 Aug 2. Antioxid Redox Signal. 2013. PMID: 22703297 Free PMC article. Review. - Oxidative status of cardinal ligament in pelvic organ prolapse.
Fang G, Hong L, Liu C, Yang Q, Zhang Q, Li Y, Li B, Wu D, Wu W, Shi H. Fang G, et al. Exp Ther Med. 2018 Oct;16(4):3293-3302. doi: 10.3892/etm.2018.6633. Epub 2018 Aug 21. Exp Ther Med. 2018. PMID: 30250520 Free PMC article.
References
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. - PubMed
- Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. - PubMed
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. - PubMed
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases