A panel of artificial APCs expressing prevalent HLA alleles permits generation of cytotoxic T cells specific for both dominant and subdominant viral epitopes for adoptive therapy - PubMed (original) (raw)
Comparative Study
. 2009 Aug 15;183(4):2837-50.
doi: 10.4049/jimmunol.0804178. Epub 2009 Jul 27.
Affiliations
- PMID: 19635907
- PMCID: PMC3079474
- DOI: 10.4049/jimmunol.0804178
Comparative Study
A panel of artificial APCs expressing prevalent HLA alleles permits generation of cytotoxic T cells specific for both dominant and subdominant viral epitopes for adoptive therapy
Aisha N Hasan et al. J Immunol. 2009.
Abstract
Adoptive transfer of virus-specific T cells can treat infections complicating allogeneic hematopoietic cell transplants. However, autologous APCs are often limited in supply. In this study, we describe a panel of artificial APCs (AAPCs) consisting of murine 3T3 cells transduced to express human B7.1, ICAM-1, and LFA-3 that each stably express one of a series of six common HLA class I alleles. In comparative analyses, T cells sensitized with AAPCs expressing a shared HLA allele or autologous APCs loaded with a pool of 15-mer spanning the sequence of CMVpp65 produced similar yields of HLA-restricted CMVpp65-specific T cells; significantly higher yields could be achieved by sensitization with AAPCs transduced to express the CMVpp65 protein. T cells generated were CD8(+), IFN-gamma(+), and exhibited HLA-restricted CMVpp65-specific cytotoxicity. T cells sensitized with either peptide-loaded or transduced AAPCs recognized epitopes presented by each HLA allele known to be immunogenic in humans. Sensitization with AAPCs also permitted expansion of IFN-gamma(+) cytotoxic effector cells against subdominant epitopes that were either absent or in low frequencies in T cells sensitized with autologous APCs. This replenishable panel of AAPCs can be used for immediate sensitization and expansion of virus-specific T cells of desired HLA restriction for adoptive immunotherapy. It may be of particular value for recipients of transplants from HLA-disparate donors.
Figures
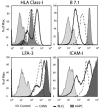
Fig.1. AAPC demonstrate comparable expression of HLA and Co-stimulatory molecules to CAMs and BLCL
HLA expression is shown for A*0201 AAPCs compared to CAM and BLCL generated from an HLA- A*0201 bearing donor using one HLA- A*0201 specific Ab. Co-stimulatory molecule expression on each cell type is also shown. AAPCs demonstrate comparable or higher expression of the HLA and co-stimulatory molecules.
Fig 2. Comparative HLA-Tetramer analysis of T cells sensitized using CMVpp65 peptide PL AAPC class I vs AAPC class I-pp65
(A) Tetramer analysis (FACS) performed at inception (day 0) and after 21 days of sensitization is shown for a single donor from each of the 3 groups for which HLA- CMVpp65 peptide tetramers were available (HLA- A*0201, A*2402 and B*0702). X axis= CD8 and Y axis= CMV-tetramer. Significant expansion of CMV peptide-specific T cells was seen in all cultures, while AAPC class I-pp65 induced comparable or higher proportions of tetramer positive CD8 + T cells. (20% Vs 22% for HLA- B*0702 donor, 6.5% Vs 4% for HLA- A*2402 donor and 91% vs. 67% for HLA- A*0201 donor). (B) Degree of expansion and (C) Absolute numbers of CD8+ tetramer positive T cells generated using autologous CMVpp65 peptide loaded (PL) CAM and AAPC class I (○) were closely correlated and were significantly lower than the number of tetramer positive T cells generated using AAPC class I-pp65 (●). (p <0.01 for HLA- A*0201 donors for sensitization with autologous CAMs vs sensitization with AAPC A2-pp65 and p < 0.03 for sensitization with PL A2 AAPCs vs AAPCA2-pp65).  , tetramer positive CD8 T cells for control TC cultures sensitized without peptide.
, tetramer positive CD8 T cells for control TC cultures sensitized without peptide.
Fig 3. Phenotype of Tetramer Positive CMV-specific T cells
(A) T cells from a donor bearing HLA- A*0201 were analyzed at different time points during sensitization using either PL AAPC A0201 or AAPC A0201pp65 to characterize the memory TC phenotype. CD8+ NLV-Tet + T cells shown ( x axis= CD45 RA and y axis= CD62L ). By day 17-24, all tetramer positive T cells sensitized using either PL AAPC A0201 or AAPCA0201pp65 were effector memory in phenotype (negative for CD45RA & CD62L). (B) TCR Vβ repertoire is shown for tetramer positive T cells from an HLA- A*0201 donor. Each bar graph represents the % CD8+ tetramer+ T-cells in each of the 24 TCR Vb subfamilies. T cells sensitized with both PL CAMs (upper graph) and HLA- A*0201+ AAPC class I-pp65 (lower graph) demonstrate predominant usage of Vb 14 subfamily.
Fig 4. Comparative analysis of IFNβ+ T-cells and HLA restricted cytotoxicity for TC generated using autologous CAMs or AAPCs expressing single HLA alleles
(A) CMV-specific TC generated using either CAM or AAPCs, were quantitated by an intracellular IFN-β assay {PL CAMs or AAPCclassI (○) , AAPCclass I-pp65 (●), CAM or AAPC without peptide (□)}. Sensitization with AAPC class I-pp65, expressing each HLA allele, resulted in higher yields of IFNβ+ T-cells compared to sensitization using PL AAPC class I for all donors (p= 0.03 for HLA- A*0201 donors). (B) Cytotoxicity against PL single HLA sharing EBV-BLCL lines is shown after 21 days of sensitization for all cultures (E:T =20:1). CAM or AAPC without peptide = (□), PL CAM or AAPC class I = (○) and AAPC class I-pp65 = (●), PL HLA mismatched BLCL ( ). PL AAPC class I or AAPC Class I-pp65 induced T cells with comparable cytotoxicity to T cells sensitized with PL CAMs when tested against EBV BLCL sharing the immunodominant HLA allele (A*0201, B*0702 or A*2402). Cytotoxicity against subdominant epitopes presented by C*0401 or B*0801 was higher and significant for T-cells sensitized with AAPC in comparison to TC sensitized with PL CAMs, which show minimal lysis of these targets that falls within the range of controls. (C) The proportion of CD8+ IFNβ+ T-cells generated using AAPC class I-pp65 was significantly correlated with the in-vitro cytotoxicity against PL autologous EBV-BLCLs for the respective T-cell lines (r=0.53, P < 0,001). The % cytotoxicity for T-cell lines sensitized with PL CAMs against PL autologous EBV-BLCLs was also correlated with the percentage of IFN-β-producing T-cells, although to a lesser degree (r=0.44, p = 0.01)
). PL AAPC class I or AAPC Class I-pp65 induced T cells with comparable cytotoxicity to T cells sensitized with PL CAMs when tested against EBV BLCL sharing the immunodominant HLA allele (A*0201, B*0702 or A*2402). Cytotoxicity against subdominant epitopes presented by C*0401 or B*0801 was higher and significant for T-cells sensitized with AAPC in comparison to TC sensitized with PL CAMs, which show minimal lysis of these targets that falls within the range of controls. (C) The proportion of CD8+ IFNβ+ T-cells generated using AAPC class I-pp65 was significantly correlated with the in-vitro cytotoxicity against PL autologous EBV-BLCLs for the respective T-cell lines (r=0.53, P < 0,001). The % cytotoxicity for T-cell lines sensitized with PL CAMs against PL autologous EBV-BLCLs was also correlated with the percentage of IFN-β-producing T-cells, although to a lesser degree (r=0.44, p = 0.01)
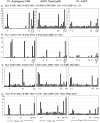
Fig 5. Mapping of Epitopes of CMVpp65 Eliciting IFNβ+ T-cell Response after Sensitization with Peptide Pool Loaded Autologous CAMs, Pool Loaded AAPC Class-I or AAPC Class-Ipp65
IFNβ+ CD8+ TC generated in response to individual subpools of CMV pp65 peptides in an epitope mapping matrix (X axis) used to identify CMVpp65 epitopes. T cells were analyzed after sensitization with (Left to Right) PL CAM or with AAPCclass-I pp65 or PL AAPC class-I expressing one HLA allele shared by the donor (Y axis = % IFNβ+ CD8+ TC, First left bar = PBMC without peptide). HLA genotype of each donor is shown above each set. (A) CD8+ IFNβ + T-cells generated using PL CAM from a donor bearing both HLA A*0201 and A*2402, were responsive to pools 3 & 23 which share NLVPMVATV, an HLA- A*0201 epitope but minimally responsive to pools 1,2 and 20 (QYDPVAALF), an A*2402 epitope. PL AAPC A2402 and AAPC A2402pp65, induced IFNβ+ CD8 TC responses to pools 1,2 and 20 as well as QYDPVAALF but not to pools 3 & 23 or the NLVPMVATV nonamer. (B) PL CAM sensitized T-cells were responsive to pools 6,7 and 18 containing RPHERNGFTV, an HLA- B*0702 epitope; AAPC B0702 pp65 induced responses to pools 9 & 21 containing TPRVTGGGAM, another known HLA- B*0702 epitope, but not to pools 6,7 & 18, while PL AAPC B0702 induced IFNβ+ TC against pools 9 & 21(TPRVTGGGAM), and pools 7 & 18 (RPHERNGFTV), both known epitopes of B0702. (C) PL CAM sensitized T-cells were responsive to pools 4 & 15 containing a peptide MSIYVYALPLKMLNI, presented by HLA- A*6801. PL AAPC C0401 and AAPC C0401pp65 induced IFN γ + CD8 TC against pools 1,2 & 20 (QYDPVAALF), an epitope presented by HLA- C*0401. (D) PL CAM sensitized T-cells were responsive to pools 3, 4 & 13 containing GPISGHVLK, a peptide presented by HLA- A*1101. PL AAPC B0801 or AAPC B0801pp65 induced IFN γ+ CD8+ TC against pools 3, 4 & 18 containing LTMTRNPQP, an epitope predicted to be presented by HLA- B*0801 in peptides 63, 64) and 3& 23 containing NLVPMVATV.
Fig 6. AAPC can be used to generate CMV-specific TC of desired HLA Restriction
(A) CMV-specific TC frequency from a donor co-expressing HLA- A*0201 and B*0702 at inception of TC culture (day 0), demonstrated 0.3% HLA- B*0702 tetramer + CD8+ TC only. The dominant T cell response induced by PL CAM was to a peptide, TPRVTGGGAM, presented by B*0702 (35% B7 tetramer+ CD8+ TC), with a subdominant response to the HLA- A*0201presented peptide NLVPMVATV ( 14% A2 tetramer+ CD8+ TC). AAPC B0702pp65 stimulated TC contained only B*0702 tetramer + CD8+ TC (20%) and no A*0201 tetramer+ TC. In contrast, AAPC A0201pp65 induced only A*0201 tetramer+ TC (7% ) and no B*0702 tetramer+ TC. (B) IFN γ+ CD8+ TC generated using (L) PL CAM were responsive to pools 6, 7 & 18 (RPHERNGFTV) a B7 epitope, and pools 8,9 & 21 (TPRVTGGGAM), also a B7 epitope with minimal response to pool 3 & 23 (NLVPMVATV), an A2 epitope, (middle) AAPC B0702pp65 were responsive only to pools 6, 7 & 18 (B7-RPHERNGFTV), and pools 9 & 21 (B7-TPRVTGGGAM), but not to pools containing A2 epitopes (pools 3 & 23) and (R) AAPC A0201pp65 generated responses to pools 3 & 23(A2-NLVPMVATV), and not to pools containing B7 epitopes (pools 6-9,18 and 21). (C) T cells sensitized using (left) PL CAM lysed only B*0702 matched peptide loaded targets, (middle) AAPC B0702pp65 lysed only against B*0702 matched targets and not against HLA A0201 matched targets (right) AAPC A0201pp65 lysed only HLA- A*0201 matched targets and not HLA- B*0702 matched targets.
Similar articles
- Artificial antigen presenting cells that express prevalent HLA alleles: A step towards the broad application of antigen-specific adoptive cell therapies.
Hasan AN, Selvakumar A, Doubrovina E, Riviere I, Sadelain MW, O'Reilly RJ. Hasan AN, et al. Discov Med. 2009 Dec;8(43):210-8. Discov Med. 2009. PMID: 20040272 Review. - Soluble recombinant CMVpp65 spanning multiple HLA alleles for reconstitution of antiviral CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation.
Paine A, Oelke M, Tischer S, Heuft HG, Blasczyk R, Eiz-Vesper B. Paine A, et al. J Immunother. 2010 Jan;33(1):60-72. doi: 10.1097/CJI.0b013e3181b56dcc. J Immunother. 2010. PMID: 19952955 - Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells.
Dupont J, Latouche JB, Ma C, Sadelain M. Dupont J, et al. Cancer Res. 2005 Jun 15;65(12):5417-27. doi: 10.1158/0008-5472.CAN-04-2991. Cancer Res. 2005. PMID: 15958591 - Use of a lentiviral vector encoding a HCMV-chimeric IE1-pp65 protein for epitope identification in HLA-Transgenic mice and for ex vivo stimulation and expansion of CD8(+) cytotoxic T cells from human peripheral blood cells.
Rohrlich PS, Cardinaud S, Lulè J, Montero-Julian FA, Prodhomme V, Firat H, Davignon JL, Perret E, Monseaux S, Necker A, Michelson S, Lemonnier FA, Charneau P, Davrinche C. Rohrlich PS, et al. Hum Immunol. 2004 May;65(5):514-22. doi: 10.1016/j.humimm.2004.02.018. Hum Immunol. 2004. PMID: 15172452 - Novel strategies for adoptive therapy following HLA disparate transplants.
O'Reilly RJ, Hasan A, Doubrovina E, Koehne G, Prockop S. O'Reilly RJ, et al. Best Pract Res Clin Haematol. 2011 Sep;24(3):381-91. doi: 10.1016/j.beha.2011.06.001. Best Pract Res Clin Haematol. 2011. PMID: 21925091 Free PMC article. Review.
Cited by
- Identification of Naturally Processed Epitope Region Using Artificial APC Expressing a Single HLA Class I Allotype and mRNA of HCMV pp65 Antigen Fragments.
Pyo HS, Hong CH, Choi H, Baek IC, Kim TG. Pyo HS, et al. Vaccines (Basel). 2022 May 16;10(5):787. doi: 10.3390/vaccines10050787. Vaccines (Basel). 2022. PMID: 35632543 Free PMC article. - Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia.
Koehne G, Hasan A, Doubrovina E, Prockop S, Tyler E, Wasilewski G, O'Reilly RJ. Koehne G, et al. Biol Blood Marrow Transplant. 2015 Sep;21(9):1663-78. doi: 10.1016/j.bbmt.2015.05.015. Epub 2015 May 29. Biol Blood Marrow Transplant. 2015. PMID: 26028505 Free PMC article. Clinical Trial. - Enhancement of the antigen-specific cytotoxic T lymphocyte-inducing ability in the PMDC11 leukemic plasmacytoid dendritic cell line via lentiviral vector-mediated transduction of the caTLR4 gene.
Iwabuchi M, Narita M, Uchiyama T, Iwaya S, Oiwa E, Nishizawa Y, Hashimoto S, Bonehill A, Kasahara N, Takizawa J, Takahashi M. Iwabuchi M, et al. Mol Med Rep. 2015 Aug;12(2):2443-50. doi: 10.3892/mmr.2015.3685. Epub 2015 Apr 24. Mol Med Rep. 2015. PMID: 25936433 Free PMC article. - Generation of Antitumor T Cells For Adoptive Cell Therapy With Artificial Antigen Presenting Cells.
Shrestha B, Zhang Y, Yu B, Li G, Boucher JC, Beatty NJ, Tsai HC, Wang X, Mishra A, Sweet K, Lancet JE, Kelley L, Davila ML. Shrestha B, et al. J Immunother. 2020 Apr;43(3):79-88. doi: 10.1097/CJI.0000000000000306. J Immunother. 2020. PMID: 31834208 Free PMC article. - Adoptive T cell therapy of cancer.
Brenner MK, Heslop HE. Brenner MK, et al. Curr Opin Immunol. 2010 Apr;22(2):251-7. doi: 10.1016/j.coi.2010.01.020. Epub 2010 Feb 17. Curr Opin Immunol. 2010. PMID: 20171074 Free PMC article. Review.
References
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. The New England journal of medicine. 1995;333:1038–1044. - PubMed
- Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. - PubMed
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–3922. - PubMed
- O'Reilly RJ, Doubrovina E, Trivedi D, Hasan A, Kollen W, Koehne G. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants. Immunologic research. 2007;38:237–250. - PubMed
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous