IDO activates regulatory T cells and blocks their conversion into Th17-like T cells - PubMed (original) (raw)
Comparative Study
. 2009 Aug 15;183(4):2475-83.
doi: 10.4049/jimmunol.0900986. Epub 2009 Jul 27.
Affiliations
- PMID: 19635913
- PMCID: PMC3677163
- DOI: 10.4049/jimmunol.0900986
Comparative Study
IDO activates regulatory T cells and blocks their conversion into Th17-like T cells
Babak Baban et al. J Immunol. 2009.
Abstract
TLR ligands are effective vaccine adjuvants because they stimulate robust proinflammatory and immune effector responses and they abrogate suppression mediated by regulatory T cells (Tregs). Paradoxically, systemic administration of high doses of CpGs that bind to TLR9 ligands stimulated Tregs in mouse spleen to acquire potent suppressor activity dependent on interactions between programmed death-1 and its ligands. This response to CpG treatment manifested 8-12 h and was mediated by a rare population of plasmacytoid dendritic cells (CD19(+) pDC) induced to express the immunosuppressive enzyme IDO after TLR9 ligation. When IDO was blocked, CpG treatment did not activate Tregs, but instead stimulated pDCs to uniformly express the proinflammatory cytokine IL-6, which in turn reprogrammed Foxp3-lineage Tregs to express IL-17. Thus, CpG-induced IDO activity in pDCs acted as a pivotal molecular switch that induced Tregs to acquire a stable suppressor phenotype, while simultaneously blocking CpG-induced IL-6 expression required to reprogram Tregs to become Th17-like effector T cells. These findings support the hypothesis that IDO dominantly controls the functional status of Tregs in response to inflammatory stimuli in physiological settings.
Figures
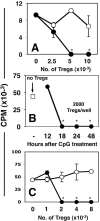
Figure 1. Systemic high dose CpG treatment rapidly activates Tregs
A. CBA mice were treated with 50μg (i/v) #1826 CpGs (●) or control #2138 (○) ODNs. After 24 hours graded numbers of FACS-sorted splenic Tregs were added to cultures containing 105 responder T cells (BM3) and APCs from CBK transgenic mice, as described in Methods. Thymidine incorporation was assessed after 72 hrs. B. MACS-enriched Tregs from B6 mice treated with CpGs (100μg, i/v) for the times indicated were added (2000/well) to cultures containing 105 responder T cells (A1), CBA female APCs and cognate (H-Y) peptide. C. Graded numbers of FACS-sorted Tregs from CpG-treated B6 mice (100μg, i/v) cultured with A1 responders and CBA APCs in the absence (●), or presence (○) of a cocktail of anti-PD1, anti-PD-L1 and anti-PD-L2 mAbs. T cell proliferation was assessed after 72 hrs. Data are the mean of triplicate cultures, and are representative of experiments performed on over five (A, C) or two (B) separate occasions. Asterisks highlight significant suppression mediated by Tregs from CpG-treated mice relative to cultures that did not contain sorted Tregs (p <0.001).
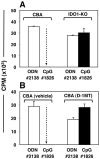
Figure 2. IDO activity is essential to activate Tregs after CpG treatment
Mice were treated with 50μg CpGs (i/v, #1826) or control ODNs (#2138). After 24 hours, FACS-sorted splenic Tregs (5000/well) were added to readout assays containing 105 responder BM3 T cells and APCs from CBK mice. A. CBA or IDO1-KO mice were used as sources of Tregs. B. Tregs were obtained from mice pre-treated for 48 hours with pellets containing vehicle alone or pellets impregnated with IDO inhibitor (D-1MT) before CpG treatment. Thymidine incorporation was assessed after 72 hours. Control cultures containing no sorted Tregs yielded 46+/-1.3 (A) and 31+/-2.5 (b) ×103 CPM respectively. Dotted arrows highlight potent suppression mediated by Tregs from CpG-treated CBA IDO-sufficient mice. Data are the mean of triplicate cultures (+/- 1sd), and are representative of experiments performed on two or more occasions.
Figure 3. Requirements for TLR9-mediated Treg activation
Sorted splenic Tregs from CpG-treated (24 hrs) mice were added to A1 T cell suppressor assays with (○) or without (●) PD-1/PD-L blocking mAbs (as in Fig. 1C). Sorted Tregs were obtained from CpG-treated (A) BALB/c, (B) IFNAR-KO, (C) IFNγR-KO, (D) GCN2-KO, and (E) CHOP-KO mice. Data shown are representative of experiments performed on two or more occasions. Sorted Tregs from CpG-treated gene-deficient and WT mice with matched backgrounds were analyzed in parallel (as in panels A & B) as positive controls for CpG-induced suppressor activity within each experiment.
Figure 4. IDO-activated Tregs block alloreactive T cell responses in vivo
A. Sorted Tregs from donor mice were mixed with MACS enriched CFSE-labeled OT-1 (Thy1.1) CD8+ T cells, and co-injected into Act-mOVA recipients. B-F. CFSE staining profiles of donor OT-1 (gated Thy1.1, CD8+) T cells analyzed 96 hours after co-adoptive transfers with splenic Tregs from the donors indicated. Markers were set to include 95% of OT-1 T cells injected alone into Act-mOVA recipients (as in C). G, H. Total numbers of OT-1 T cells detected in recipient spleens of the mice indicated. Data shown in (B-F) are representative of three mice/group, and (G, H) are the means for each group. Experiments were performed on two or more occasions.
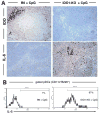
Figure 5. IDO blocks IL-6 production following high-dose CpG treatment
A. Spleen sections from CpG-treated B6 and IDO1-KO mice were stained with anti-IDO and anti-IL-6 Abs 24 hours after injecting CpGs (original magnifications, ×200). B. Splenic pDCs (gated CD11c+B220+ cells) from CpG-treated B6 (left) and IDO1-KO (right) mice were analyzed to detect intracellular IL-6 expression; markers exclude 95% of pDCs stained with isotype control mAbs, and percentages are the proportions of gated pDCs falling within this marker.
Figure 6. TLR ligation stimulates Tregs to express IL-17 when IDO is inactive
FACS analyses to detect IL-17+ cells after CpG treatment, except where indicated analyses were performed 24 hours after CpG treatment. A, B Analyses of total splenocytes from the mouse strains indicated; percentages show the proportion of CD4+ cells expressing IL-17. C. Analyses of gated CD4+ cells; percentages show the proportion of CD4+ cells co-expressing CD25 and IL-17. D. Analyses of total splenocytes from Foxp3GFP mice treated with CpGs. E. FACS-sorted GFP+ cells from Foxp3GFP donors were injected into B6 and IDO1-KO recipients two days before CpG treatment. Histograms show IL-17 staining profiles for gated GFP+ cells from spleen of CpG-treated recipient mice; the marker excludes 95% of cells stained with isotype control mAb. Data shown are representative of two or more experiments.
Figure 7. IL-6 is required to re-program Tregs into T cells expressing pro-inflammatory cytokines in the absence of IDO
A-D. FACS analyses of gated CD4 splenocytes from the CpG-treated mice indicated to detect intracellular IL-17 expression 24 hours after CpG treatment. Where indicated, mice were given oral D-1MT two days before CpG treatment. Percentages indicate the proportions of CD4+ T cells from CpG-treated mice co-expressing CD25 and IL-17. E. FACS analyses to detect intracellular expression of the cytokines indicated by gated GFP+ Tregs from untreated Foxp3GFP mice and CpG-treated Foxp3GFP mice pre-treated with D-1MT. Numbers on histograms indicate mean fluorescence intensities of total gated GFP+ cells. Percentages indicate the proportions of GFP+ cells from treated mice expressing cytokines relative to staining profiles obtained using isotype control mAbs (not shown). Data shown are representative of experiments performed on three or more occasions.
Similar articles
- Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes.
Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Sharma MD, et al. Blood. 2009 Jun 11;113(24):6102-11. doi: 10.1182/blood-2008-12-195354. Epub 2009 Apr 14. Blood. 2009. PMID: 19366986 Free PMC article. - Physiologic control of IDO competence in splenic dendritic cells.
Baban B, Chandler PR, Johnson BA 3rd, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. Baban B, et al. J Immunol. 2011 Sep 1;187(5):2329-35. doi: 10.4049/jimmunol.1100276. Epub 2011 Aug 3. J Immunol. 2011. PMID: 21813777 Free PMC article. - Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase.
Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Sharma MD, et al. J Clin Invest. 2007 Sep;117(9):2570-82. doi: 10.1172/JCI31911. J Clin Invest. 2007. PMID: 17710230 Free PMC article. - Anti-regulatory T cells.
Andersen MH. Andersen MH. Semin Immunopathol. 2017 Apr;39(3):317-326. doi: 10.1007/s00281-016-0593-x. Epub 2016 Sep 27. Semin Immunopathol. 2017. PMID: 27677755 Review. - T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.
Kahler DJ, Mellor AL. Kahler DJ, et al. Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review.
Cited by
- Contribution of tryptophan and its metabolites to transplant outcome: a mini-review.
Donoso-Meneses D, Padilla C, Moya-Guzmán MJ, Alegre ML, Pino-Lagos K. Donoso-Meneses D, et al. Front Immunol. 2024 Oct 15;15:1395421. doi: 10.3389/fimmu.2024.1395421. eCollection 2024. Front Immunol. 2024. PMID: 39474416 Free PMC article. Review. - The roles of arginases and arginine in immunity.
Canè S, Geiger R, Bronte V. Canè S, et al. Nat Rev Immunol. 2024 Oct 17. doi: 10.1038/s41577-024-01098-2. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39420221 Review. - General control nonderepressible 2 (GCN2) as a therapeutic target in age-related diseases.
Altintas O, MacArthur MR. Altintas O, et al. Front Aging. 2024 Sep 10;5:1447370. doi: 10.3389/fragi.2024.1447370. eCollection 2024. Front Aging. 2024. PMID: 39319345 Free PMC article. Review. - mRNA-delivery of IDO1 suppresses T cell-mediated autoimmunity.
Kenney LL, Chiu RS, Dutra MN, Wactor A, Honan C, Shelerud L, Corrigan JJ, Yu K, Ferrari JD, Jeffrey KL, Huang E, Stein PL. Kenney LL, et al. Cell Rep Med. 2024 Sep 17;5(9):101717. doi: 10.1016/j.xcrm.2024.101717. Epub 2024 Sep 6. Cell Rep Med. 2024. PMID: 39243754 Free PMC article. - Targeting amino acid-metabolizing enzymes for cancer immunotherapy.
Grobben Y. Grobben Y. Front Immunol. 2024 Aug 14;15:1440269. doi: 10.3389/fimmu.2024.1440269. eCollection 2024. Front Immunol. 2024. PMID: 39211039 Free PMC article. Review.
References
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002;20:197–216. - PubMed
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. - PubMed
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. - PubMed
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. - PubMed
- Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD041187/HD/NICHD NIH HHS/United States
- CA096651/CA/NCI NIH HHS/United States
- R01 CA103320/CA/NCI NIH HHS/United States
- R01 CA096651/CA/NCI NIH HHS/United States
- AI063402/AI/NIAID NIH HHS/United States
- HD041187/HD/NICHD NIH HHS/United States
- CA112431/CA/NCI NIH HHS/United States
- CA103320/CA/NCI NIH HHS/United States
- R01 AI044219/AI/NIAID NIH HHS/United States
- R01 AI063402/AI/NIAID NIH HHS/United States
- R01 CA112431/CA/NCI NIH HHS/United States
- R01 AI075165/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials