A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation - PubMed (original) (raw)
A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation
Nhat-Long L Pham et al. J Immunol. 2009.
Abstract
Inflammatory cytokines induced by infection or vaccination with adjuvant act directly or indirectly on CD8 T cells to modulate their expansion, contraction, and acquisition of memory characteristics. Importantly, the initial exposure of naive T cells to inflammatory cytokines may occur before, during, or after their interaction with stimulating dendritic cells (DC) and it is unknown whether and how the timing of cytokine exposure impacts the CD8 T cell response. In this study, we use an immunization strategy with peptide-coated mature DC that, in the absence of inflammatory cytokines, results in a transient effector phase followed by the accelerated acquisition of memory characteristics by the responding CD8 T cells. Induction of inflammatory cytokines by TLR agonists, at the time of DC immunization or 2-4 days after DC immunization, prevented the early acquisition of memory characteristics by the responding CD8 T cells. Interestingly, although induction of inflammatory cytokines at the time of DC immunization increased the effector response, induction of inflammatory cytokines after DC immunization did not promote further expansion of the responding CD8 T cells but still prevented their early acquisition of memory characteristics. In contrast, induction of inflammatory cytokines 2 days before DC immunization did not prevent the CD8 T cells from early acquisition of memory characteristics. Furthermore, TLR ligand-induced inflammatory cytokines had the most significant impact on the phenotype and function of proliferating CD8 T cells. These data suggest that a default pathway of memory CD8 T cell differentiation is deflected toward sustained effector commitment by encounter with inflammatory cytokines during Ag-driven proliferation.
Figures
Figure 1
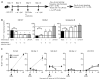
Low number OT-1 adoptive transfer mimics the endogenous CD8 T cell response after peptide-coated DC immunization and Listeria infection. Naïve B6 Thy1.2 mice received ~ 500 naïve Thy1.1 OT-1 cells and were immunized with ~ 1×106 Ova257-264-coated DC ± CpG (100 μg IP) or infected with ~1×105 cfu LM-Ova. (A) Recovery of Flt3L-expanded, in vivo LPS stimulated splenic dendritic cells (DC) and their activation profile after isolation and prior to immunization. Frequency (mean ± S.D., N=3) as detected by ICS for IFN-γ (all Ova-specific cells) and Thy1.1 expression (OT-1 cells) in the spleen (B) or direct ex-vivo tetramer staining (all Ova-specific cells) and Thy1.1 expression (OT-1 cells) in both the peripheral blood and spleen (C) on day 7 post DC immunization in the presence or absence of CpG. (D) Total number (mean ± S.D., N=3) of endogenous Ova257-specific CD8 T cells and OT-1 in the spleen as detected by ICS on day 7 post DC immunization. (E) Representative histograms and (F) Cumulative data representing CD127, KLRG-1, and Granzyme B expression on OT-1 cells and endogenous Ova257-specific CD8 T cells as detected either by ICS or direct ex-vivo tetramer staining in DC immunization in the presence or absence of CpG-induced inflammation and LM-Ova infection. Numbers in histograms represent frequency (mean ± S.D., N=3) of cells that are positive for indicated markers. Shaded histograms represent isotype-control staining. Data are representative of at least 3 experiments. N.D. = not done.
Figure 2
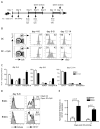
CpG ODN does not signal directly on transferred DC or T cells but rather induces the inflammatory environment that influences the rate of memory generation following DC immunization. (A) Experimental design: naïve wt or TLR9−/− B6 Thy1.2 mice received ~ 500 naïve Thy1.1 OT-1 cells. Mice were immunized with either ~ 1×106 Ova257-264-coated wt DC or TLR9−/− DC with or without co-injection of CpG (100 μg IP). OT-1 cells from PBL were analyzed on day +7 post immunization or infection. Mice were then challenged with early booster infection with actA-deficient LM-Ova and frequency of OT-1 was analyzed 5 days later. (B) Recovery of Flt3L-expanded, in vivo LPS stimulated wt and TLR9−/− splenic dendritic cells (DC) and their activation profile after isolation and prior to immunization. (C) Percentage (mean ± S.D., N≥3) of OT-1 in PBL expressing CD127 and KLRG-1 in both wt and TLR9−/− recipients at day 7 post priming with either wt or TLR9−/− DC in the presence or absence of CpG co-injection. (D) Frequency of OT-1 in PBL in wild-type recipients at day 7 post priming with either wt or TLR9−/− DC in the presence or absence of CpG and at day 5 following early booster infection with actA-deficient LM-Ova. (E) Frequency of OT-1 in PBL in TLR9−/− recipients at day 7 post priming with either wt DC in the presence or absence of CpG and at day 5 following early booster infection with actA-deficient LM-Ova. Data are representative of at least 2 independent experiments.
Figure 3
CpG-induced inflammatory environment in the host displayed unique cytokine profiles and kinetics. (A) Experimental design: naïve B6 Thy1.2 mice received ~ 500 naïve Thy1.1 OT-1 cells. Mice were immunized with ~ 1×106 Ova257-264-coated DC in the presence or absence of CpG co-injection (~100 μg IP). Sera were obtained via retro-orbital bleeding at 6, 12, 24, and 48 hrs after DC immunization. OT-1 cells from PBL were analyzed on day +7 post immunization or infection. Mice were then challenged with early booster infection with actA-deficient LM-Ova and frequency of OT-1 was analyzed 5 days later. (B) Serum IL-12(p70), IL-10, IL-6, IFN-γ and GM-CSF from DC and DC + CpG immunization groups were measured using Bio-Plex Mouse Cytokines Assays (Bio-Rad). Serum from naïve B6 mice served to determine the cytokine level at time 0. Data presented as mean ± S.D., N=4. (C) Percentage (mean ± S.D., N=4) of OT-1 in PBL expressing CD127 and KLRG-1 at day 7 post priming with DC in the presence or absence of CpG co-injection. (D) Frequency of OT-1 in PBL at day 7 post priming with DC in the presence or absence of CpG and at day 5 following early booster infection with actA-deficient LM-Ova.
Figure 4
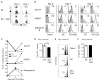
Exposure to inflammation prior to antigen-driven expansion of CD8 T cells does not prevent accelerated memory generation after DC immunization. (A) Experimental design: naïve B6 Thy1.2 mice received ~ 500 naïve Thy1.1 OT-1 cells at day –3 relative to DC immunization. Inflammation was induced by CpG at day −2, day 0, or day +2 in the indicated groups while other groups did not receive CpG. Day 0 indicates the time when all mice were immunized with ~ 1×106 Ova257-264-coated DC. An additional control group received ~1×105 cfu LM-Ova on day 0. OT-1 cells from PBL were analyzed on day +6 post immunization or infection. Half of the mice from each group then received booster infection actA-deficient LM-Ova and the frequency of OT-1 was determined at day 4 following boosting. (B) The percentage (mean ± S.D., N=3) of OT-1 cells in PBL expressing CD127, KLRG-1, and Granzyme B from the indicated groups of mice treated with CpG at different times. DC immunization and LM-Ova infection served as the controls for low inflammation and high inflammation, respectively. Statistical analysis was performed with student t-test between groups. * = statistically significant (p<0.05); n.s. = not statistically significant (p>.05) (C) OT-1 recipient mice immunized with DC-Ova and received no CpG or CpG at day −2, 0, and +2. Control mice were infected with virulent LM-Ova. Mice were boosted with actA-deficient LM-Ova on day 6 after DC-Ova immunization or LM-Ova infection. Data presented as frequency of OT-1 in PBL on different days. Data are representative of at least 3 independent experiments.
Figure 5
Inflammation exerts the greatest impact on memory differentiation during proliferation of CD8 T cells. (A) Experimental design: naïve B6 Thy1.2 mice received ~ 500 naïve Thy1.1 OT-1 cells and were immunized with Ova-coated DC one day later (day 0). BrdU was administered to individual groups of mice at three different time periods: day 4–6, day 6–8, and day 12–14. CpG was administered for some mice in each group at the beginning of each interval. Control groups received BrdU but no CpG treatment. OT-1 cells from the spleen were analyzed at day 6, 8 and 14 from the respective groups. (B) Representative histograms of BrdU incorporation by OT-1 cells at various time intervals. Numbers on the right in histograms represent the percentage of OT-1 cells incorporated BrdU during the 2-day period. (C) The percentage (mean ± S.D., N=3) of OT-1 cells in the spleen expressing CD127, KLRG-1, and Granzyme B from the DC and DC+CpG groups in the three time periods of BrdU treatment (day 4–6, day 6–8, and day 12–14). (D) Representative histograms of isotype staining and CD127 expression on BrdU+ and BrdU− fractions of OT-1 cells at day 8 post DC priming either in the presence or absence of CpG administered to the hosts on day 5. Shaded and unshaded histograms represent DC immunization and DC+CpG groups, respectively. Top two panels are from the BrdU-negative OT-1 population and bottom two panels are from the brdU-positive OT-1. Top and bottom (in parentheses) numbers indicates the frequency of OT-1 cells expressing CD127 in the absence or presence of CpG, respectively. (E) Normalized percent of reduction in CD127 expression of both BrdU-negative and BrdU-positive OT-1 populations is calculated as followed: (100% - ((% CD127 positive OT-1 on DC+CpG)/(% CD127 positive OT-1 on DC only)) on both day 7 and 8 following DC either in the presence or absence of CpG administered to the hosts on day 5. Statistical analysis was performed with student t-test.
Figure 6
The default pathway of memory CD8 T cell differentiation is deflected by encounter with inflammatory cytokines. Naïve B6 Thy1.2 mice received ~ 500 naïve Thy1.1 OT-1 cells. Mice were immunized with ~ 1×106 Ova257-264-coated DC in the presence or absence of CpG co-injection (~100 μg IP). (A) Analysis of OT-1 cells in the spleen from a representative mouse from each group (DC and DC+CpG) at day 4 and day 7 following DC immunization. (B and C) Phenotypic analysis of naïve OT-1 cells (day 0) and responding OT-1 cells at day 4 and 7 following DC immunization in the presence or absence of CpG ODN coinjection. (B) Representative histograms and (C) and kinetic of CD127, KLRG-1, and Granzyme B expression by OT-1 cells. (D) Number of total OT-1 cell recovered from the spleen of mice immunized with DC or DC+CpG; n.s. = not statistically significant by student t-test. (E and F) In vivo cytolytic assay: At day 4 following immunization with either DC or DC+CpG, OT-1 recipient mice or naïve control mice that received neither OT-1 nor DC were injected with target cells consisting of 10×106 CFSEhi-labeled B6 splenocytes (no peptide) and 10×106 CFSElo-labeled B6 splenocytes (coated with Ova257 peptide). (E) Representative histograms of CFSE-labeled cells recovered from each group at 5 hours after target cell injection. Number in histogram is percentage of CFSElo population remained after 5 hours. (F) Percent specific killing calculated by the formula described in Materials and Methods section. Cumulative data are from three mice per group.
Figure 7
Model representing how the timing of inflammation relative to DC immunization influences the rate at which CD8 T cells acquire memory characteristics. (A) After DC immunization, responding CD8 T cells go through a transient effector phase but the low inflammatory environment during the robust proliferative expansion phase promotes accelerated acquisition of memory characteristics by the responding CD8 T cells. (B) Either exposure of non-proliferating naïve CD8 T cells to inflammation prior to their interaction with stimulating DC or exposure too inflammation of non-proliferating CD8 T cells after contraction does not prevent early acquisition of memory characteristics or alter existing memory characteristics of the responding CD8 T cells. (C) During an acute infection or DC immunization + TLR agonist, heightened inflammation during the priming and/or robust proliferative expansion sustains the effector differentiation program and prevents early acquisition of memory characteristics by the responding CD8 T cells, resulting in their slow conversion to memory.
Similar articles
- Manipulating the rate of memory CD8+ T cell generation after acute infection.
Badovinac VP, Harty JT. Badovinac VP, et al. J Immunol. 2007 Jul 1;179(1):53-63. doi: 10.4049/jimmunol.179.1.53. J Immunol. 2007. PMID: 17579021 - IFN-gamma receptor signaling regulates memory CD8+ T cell differentiation.
Sercan O, Stoycheva D, Hämmerling GJ, Arnold B, Schüler T. Sercan O, et al. J Immunol. 2010 Mar 15;184(6):2855-62. doi: 10.4049/jimmunol.0902708. Epub 2010 Feb 17. J Immunol. 2010. PMID: 20164422 - Antigen presentation by an immature myeloid dendritic cell line does not cause CTL deletion in vivo, but generates CD8+ central memory-like T cells that can be rescued for full effector function.
Dumortier H, van Mierlo GJ, Egan D, van Ewijk W, Toes RE, Offringa R, Melief CJ. Dumortier H, et al. J Immunol. 2005 Jul 15;175(2):855-63. doi: 10.4049/jimmunol.175.2.855. J Immunol. 2005. PMID: 16002683 - Plasticity in programming of effector and memory CD8 T-cell formation.
Arens R, Schoenberger SP. Arens R, et al. Immunol Rev. 2010 May;235(1):190-205. doi: 10.1111/j.0105-2896.2010.00899.x. Immunol Rev. 2010. PMID: 20536564 Free PMC article. Review. - Surviving the crash: transitioning from effector to memory CD8+ T cell.
D'Cruz LM, Rubinstein MP, Goldrath AW. D'Cruz LM, et al. Semin Immunol. 2009 Apr;21(2):92-8. doi: 10.1016/j.smim.2009.02.002. Epub 2009 Mar 6. Semin Immunol. 2009. PMID: 19269192 Free PMC article. Review.
Cited by
- Vaccine adjuvant-elicited CD8+ T cell immunity is co-dependent on T-bet and FOXO1.
Ivanova DL, Thompson SB, Klarquist J, Harbell MG, Kilgore AM, Lasda EL, Hesselberth JR, Hunter CA, Kedl RM. Ivanova DL, et al. Cell Rep. 2023 Aug 29;42(8):112911. doi: 10.1016/j.celrep.2023.112911. Epub 2023 Jul 29. Cell Rep. 2023. PMID: 37516968 Free PMC article. - Nano toolbox in immune modulation and nanovaccines.
Azharuddin M, Zhu GH, Sengupta A, Hinkula J, Slater NKH, Patra HK. Azharuddin M, et al. Trends Biotechnol. 2022 Oct;40(10):1195-1212. doi: 10.1016/j.tibtech.2022.03.011. Epub 2022 Apr 19. Trends Biotechnol. 2022. PMID: 35450779 Free PMC article. Review. - Deletion of immune evasion genes provides an effective vaccine design for tumor-associated herpesviruses.
Brar G, Farhat NA, Sukhina A, Lam AK, Kim YH, Hsu T, Tong L, Lin WW, Ware CF, Blackman MA, Sun R, Wu TT. Brar G, et al. NPJ Vaccines. 2020 Nov 5;5(1):102. doi: 10.1038/s41541-020-00251-x. NPJ Vaccines. 2020. PMID: 33298958 Free PMC article. - Polyanhydride Nanoparticles Induce Low Inflammatory Dendritic Cell Activation Resulting in CD8+ T Cell Memory and Delayed Tumor Progression.
Darling R, Senapati S, Christiansen J, Liu L, Ramer-Tait AE, Narasimhan B, Wannemuehler M. Darling R, et al. Int J Nanomedicine. 2020 Sep 7;15:6579-6592. doi: 10.2147/IJN.S261041. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32982219 Free PMC article. - TCR Signal Strength and Antigen Affinity Regulate CD8+ Memory T Cells.
Solouki S, Huang W, Elmore J, Limper C, Huang F, August A. Solouki S, et al. J Immunol. 2020 Sep 1;205(5):1217-1227. doi: 10.4049/jimmunol.1901167. Epub 2020 Aug 5. J Immunol. 2020. PMID: 32759295 Free PMC article.
References
- Haring JS, V, Badovinac P, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. - PubMed
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science (New York, NY) 2000;290:1354–1358. - PubMed
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. - PubMed
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI050073/AI/NIAID NIH HHS/United States
- AI150073/AI/NIAID NIH HHS/United States
- R01 AI042767/AI/NIAID NIH HHS/United States
- R37 AI042767/AI/NIAID NIH HHS/United States
- R01 AI046653-09/AI/NIAID NIH HHS/United States
- R01 AI050073-08/AI/NIAID NIH HHS/United States
- R37 AI042767-11/AI/NIAID NIH HHS/United States
- AI42767/AI/NIAID NIH HHS/United States
- R21 AI042767/AI/NIAID NIH HHS/United States
- AI46653/AI/NIAID NIH HHS/United States
- R01 AI046653/AI/NIAID NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials