Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Sep 1;27(25):4109-15.
doi: 10.1200/JCO.2009.21.9527. Epub 2009 Jul 27.
Affiliations
- PMID: 19636001
- PMCID: PMC2734422
- DOI: 10.1200/JCO.2009.21.9527
Randomized Controlled Trial
Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial
Hanna K Sanoff et al. J Clin Oncol. 2009.
Abstract
Purpose: Racial disparities in colorectal cancer (CRC) survival are documented, but there are few data on comparative response to chemotherapy. A subgroup analysis of a multisite National Cancer Institute-sponsored trial (N9741) was performed comparing outcomes of black and white patients with metastatic CRC receiving uniform treatment.
Patients and methods: Adverse events (AEs), response rate (RR), time to progression (TTP), overall survival (OS), and dose-intensity were examined as a function of self-reported race in 1,412 patients treated with irinotecan/fluorouracil, fluorouracil/oxaliplatin, or irinotecan/oxaliplatin. Pharmacogenetic analysis was performed on 486 patients with blood available for germline DNA analysis.
Results: OS was 1.5 months shorter and TTP was 0.6 months shorter in black than white patients (OS: hazard ratio [HR] = 1.13; 95% CI, 0.90 to 1.42; TTP: HR = 0.91, 95% CI, 0.73 to 1.13); neither difference was statistically significant. RR was significantly higher in whites (41%) than blacks (28%; P = .008). Grade 3 or greater AEs were also higher in whites (48%) than blacks (34%; P = .004). These relationships were maintained in multivariate models adjusting for arm, age, sex, and performance status. There was no difference in dose-intensity of delivered therapy. Significant racial differences in prevalence of pharmacogenetic variants were observed, although small sample size precluded investigating the relationship between treatment, race, and genotype.
Conclusion: OS and TTP are similar in black and white patients treated per protocol with standardized therapy for metastatic CRC. However, RR and AEs vary considerably by race. The marked racial differences in relevant pharmacogenetics, a potential explanation for differing RR and AEs, are worthy of future study.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
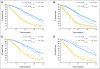
Fig 1.
Kaplan-Meier plots of time to progression (TTP) and overall (OS) survival for (A) all patients and by arm: (B) irinotecan, fluorouracil, and leucovorin; (C) oxaliplatin, fluorouracil, and leucovorin; and (D) irinotecan and oxaliplatin.
Similar articles
- Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741.
Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Goldberg RM. Sanoff HK, et al. J Clin Oncol. 2008 Dec 10;26(35):5721-7. doi: 10.1200/JCO.2008.17.7147. Epub 2008 Nov 10. J Clin Oncol. 2008. PMID: 19001325 Free PMC article. Clinical Trial. - Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial.
Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, Berry S, Polite BN, O'Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Venook AP, et al. JAMA. 2017 Jun 20;317(23):2392-2401. doi: 10.1001/jama.2017.7105. JAMA. 2017. PMID: 28632865 Free PMC article. Clinical Trial. - A randomized, placebo-controlled phase ii study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy.
Hecht JR, Pillai M, Gollard R, Heim W, Swan F, Patel R, Dreiling L, Mo M, Malik I. Hecht JR, et al. Clin Colorectal Cancer. 2010 Apr;9(2):95-101. doi: 10.3816/CCC.2010.n.013. Clin Colorectal Cancer. 2010. PMID: 20378503 Clinical Trial. - The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
Hind D, Tappenden P, Tumur I, Eggington S, Sutcliffe P, Ryan A. Hind D, et al. Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574 Review.
Cited by
- Molecular, Socioeconomic, and Clinical Factors Affecting Racial and Ethnic Disparities in Colorectal Cancer Survival.
Yousef M, Yousef A, Chowdhury S, Fanaeian MM, Knafl M, Peterson J, Zeineddine M, Alfaro K, Zeineddine F, Goldstein D, Hornstein N, Dasari A, Huey R, Johnson B, Higbie V, Bent A, Kee B, Lee M, Morelli MP, Morris VK, Halperin D, Overman MJ, Parseghian C, Vilar E, Wolff R, Raghav KP, White MG, Uppal A, Sun R, Wang W, Kopetz S, Willis J, Shen JP. Yousef M, et al. JAMA Oncol. 2024 Sep 12:e243666. doi: 10.1001/jamaoncol.2024.3666. Online ahead of print. JAMA Oncol. 2024. PMID: 39264607 - Social Determinants of Health and the Link to Colorectal Cancer Outcomes.
Lorentsen MK, Sanoff HK. Lorentsen MK, et al. Curr Treat Options Oncol. 2024 Apr;25(4):453-464. doi: 10.1007/s11864-024-01191-7. Epub 2024 Mar 18. Curr Treat Options Oncol. 2024. PMID: 38498252 Review. - Inflammation, microbiome and colorectal cancer disparity in African-Americans: Are there bugs in the genetics?
Ahmad S, Ashktorab H, Brim H, Housseau F. Ahmad S, et al. World J Gastroenterol. 2022 Jul 7;28(25):2782-2801. doi: 10.3748/wjg.v28.i25.2782. World J Gastroenterol. 2022. PMID: 35978869 Free PMC article. Review. - The association of health insurance and race with treatment and survival in patients with metastatic colorectal cancer.
Mitsakos AT, Irish W, Parikh AA, Snyder RA. Mitsakos AT, et al. PLoS One. 2022 Feb 17;17(2):e0263818. doi: 10.1371/journal.pone.0263818. eCollection 2022. PLoS One. 2022. PMID: 35176030 Free PMC article. - Racial differences in survival and response to therapy in patients with metastatic colorectal cancer: A secondary analysis of CALGB/SWOG 80405 (Alliance A151931).
Snyder RA, He J, Le-Rademacher J, Ou FS, Dodge AB, Zemla TJ, Paskett ED, Chang GJ, Innocenti F, Blanke C, Lenz HJ, Polite BN, Venook AP. Snyder RA, et al. Cancer. 2021 Oct 15;127(20):3801-3808. doi: 10.1002/cncr.33649. Epub 2021 Aug 10. Cancer. 2021. PMID: 34374082 Free PMC article. Clinical Trial.
References
- Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. - PubMed
- Doubeni CA, Field TS, Buist DS, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109:612–620. - PubMed
- American Cancer Society. Colorectal Cancer Facts and Figures 2008-2010. Atlanta, GA: American Cancer Society; 2008.
- Irby K, Anderson WF, Henson DE, et al. Emerging and widening colorectal carcinoma disparities between blacks and whites in the United States (1975-2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–797. - PubMed
- Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87:1686–1693. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- KL2 RR025746/RR/NCRR NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical