The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells - PubMed (original) (raw)
The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells
Shawn A Milligan et al. Clin Cancer Res. 2009.
Abstract
Purpose: Activation of the c-Met and epidermal growth factor receptors (EGFR) promotes the growth and survival of non-small cell lung cancer (NSCLC). Specific receptor antagonists have shown efficacy in the clinic, but tumors often become resistant to these therapies. We investigated the ability of (-)-epigallocatechin-3-gallate (EGCG) to inhibit cell proliferation, and c-Met receptor and EGFR kinase activation in several NSCLC cell lines.
Experimental design: NSCLC cell lines with variable sensitivity to the EGFR antagonist erlotinib were studied. Cell growth was evaluated using proliferation and colony formation assays. Kinase activation was assessed via Western blot analysis. Experiments were conducted with EGCG, the EGFR antagonist erlotinib, and the c-Met inhibitor SU11274. The antagonists were also tested in a xenograft model using SCID mice.
Results: EGCG inhibited cell proliferation in erlotinib-sensitive and -resistant cell lines, including those with c-Met overexpression, and acquired resistance to erlotinib. The combination of erlotinib and EGCG resulted in greater inhibition of cell proliferation and colony formation than either agent alone. EGCG also completely inhibited ligand-induced c-Met phosphorylation and partially inhibited EGFR phosphorylation. The triple combination of EGCG/erlotinib/SU11274 resulted in a greater inhibition of proliferation than EGCG with erlotinib. Finally, the combination of EGCG and erlotinib significantly slowed the growth rate of H460 xenografts.
Conclusion: EGCG is a potent inhibitor of cell proliferation, independent of EGFR inhibition, in several NSCLC cell lines, including those resistant to both EGFR kinase inhibitors and those overexpressing c-Met. Therefore, EGCG might be a useful agent to study as an adjunct to other anticancer agents.
Conflict of interest statement
Conflict of interest statement: The authors express no conflicts of interest.
Figures
Fig 1
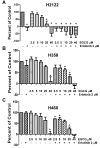
EGCG effects cell growth of NSCLC cells, and increases their sensitivity to erlotinib. Cell viability of H2122 (A), H358 (B) and H460 (C) non-small cell cancer cells in vitro in response to EGCG and erlotinib. Cells were cultured in 96-well plates in the presence of increasing concentrations of EGCG, erlotinib at 2 μM or combinations of EGCG plus erlotinib. Viability was assessed using a tetrazolium assay after allowing growth of cells for 72 h, and the change in the number of cells in treated wells were expressed as a percent relative to the changes in the number of cells in the control wells over 72 hours; normalized to 100. Negative numbers in the graph for the treated wells mean the number of cells decreased from the number of cells that were found in the control wells at the initiation of treatment (T=0). * P < 0.05 compared with the control. + P < 0.05 EGCG alone vs. EGCG as same dose plus erlotinib.
Fig 2
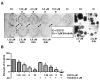
EGCG and erlotinib are more effective than either agent alone at inhibiting growth of colonies. Cells were cultured at low density and treated with EGCG, erlotinib or combinations every 48 h for 7 days. (A) Colonies were stained with crystal violet and then photographed. In (B), the crystal violet was extracted and assayed by spectrophotometry. EGCG was used at the concentrations noted in the figure and erlotinib was used at 2 μM. * P < 0.05 compared with FBS control. + P < 0.05 EGCG alone vs. EGCG as same dose plus erlotinib.
Fig 3
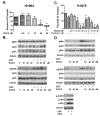
EGCG effects signaling in growth factor receptor signaling in H2111, H358 and H460 NSCLC cells. Cells were stimulated with either HGF at 33 ng/ml (A) or EGF at 100 ng/ ml (B). As noted, cells were pretreated with EGCG 10 μM to 40 μM for 4 hours (A and B) or 2 mM erlotinib for 1 hour (C). Cell lysates were collected and subjected to SDS-PAGE, western blotting and analyzed with phospho-specific antibodies to the c-Met receptor (pMet), Akt (p-Akt), Erk1/2 (pErk) and the EGFR (pEGFR). Tubulin was used as a control for loading.
Fig 4
EGCG effects cell proliferation and kinase activity in the H1993 c-Met overexpressing cell line and the H1975 T790M EGFR TKI resistant cell line. In (A) (H1993) & (C) (H1975), cells were cultured in the presence of EGCG 2.5, 5.0, 10, 20 and 40 μM, or erlotinib 2 μM or combinations as noted. Viability was assessed after 72 h using a tetrazolium based assay and the data were expressed as described in the legend to Figure 1. In (B), H1993 cells were pretreated with EGCG 10-40 μM and then left unstimulated or EGF or HGF was added. In (D), H1975 cells were pretreated with EGCG 10-40 μM and left unstimulated or stimulated with HGF at 33 ng/ml or EGF at 100 ng/ml as noted. Cells were also treated with 2 μM erlotinib prior to exposure to EGF. In (B), and (D), cell lysates were subjected to SDS-PAGE and western blotting with phospho-specific antibodies to the c-Met receptor (p-Met), Akt (p-Akt), Erk1/2 (p-Erk) and the EGFR (pEGFR). Tubulin was used as a control for loading. * P < 0.05 compared with FBS control. + P < 0.05 EGCG alone vs. EGCG as same dose plus erlotinib.
Fig 5
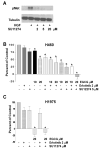
Combinations of EGCG, a c-Met inhibitor and erlotinib synergistically block proliferation of H460 cells. In (A), H460 cells were pretreated with SU11274 at varying doses as indicated prior to stimulation with HGF 33 ng/ml. Lysates were subjected to PAGE and Western blotting with a phosphospecific antibody to the c-Met receptor. Tubulin was uses as a control for loading. In (B), H460 cells were cultured in the presence of EGCG at 10 or 20 μM, erlotinib at 2μM, or SU11274 at 5 μM alone or in combination. Viability was assessed after 72 h using a tetrazolium based assay and the data were expressed as described in the legend to Figure 1. In (C), H1975 cells were cultured in the presence of erlotinib at 2 μM, SU11274 at 5 μM, EGCG at 20 μM or combinations as noted. Viability was again assessed as described in the legend to Figure 1. * P < 0.05 compared with FBS control.
Fig 6
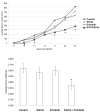
Combinations of EGCG and erlotinib are effective a slowing the growth of H460 tumors. Two million H460 cells were injected subcutaneously in male SCID mice. After 3 days, the mice were gavaged with EGCG (15/mg/kg), erlotinib (10/mg/kg), both EGCG and erlotinib or with 2% Tween-80 control as indicated. In (A), tumor volumes were measured. Data is expressed as cubic mm volume. In (B), animals were sacrificed at day 22 and tumors were dissected and weighed. * P < 0.05 EGCG + erlotinib vs. control.
Similar articles
- Synergistic inhibition of head and neck tumor growth by green tea (-)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor.
Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, Yang CS, Chen Z', Shin DM. Zhang X, et al. Int J Cancer. 2008 Sep 1;123(5):1005-14. doi: 10.1002/ijc.23585. Int J Cancer. 2008. PMID: 18546267 Free PMC article. - Combined therapy with mutant-selective EGFR inhibitor and Met kinase inhibitor for overcoming erlotinib resistance in EGFR-mutant lung cancer.
Nakagawa T, Takeuchi S, Yamada T, Nanjo S, Ishikawa D, Sano T, Kita K, Nakamura T, Matsumoto K, Suda K, Mitsudomi T, Sekido Y, Uenaka T, Yano S. Nakagawa T, et al. Mol Cancer Ther. 2012 Oct;11(10):2149-57. doi: 10.1158/1535-7163.MCT-12-0195. Epub 2012 Jul 25. Mol Cancer Ther. 2012. PMID: 22844075 - Synergistic effect of afatinib with su11274 in non-small cell lung cancer cells resistant to gefitinib or erlotinib.
Chen G, Noor A, Kronenberger P, Teugels E, Umelo IA, De Grève J. Chen G, et al. PLoS One. 2013;8(3):e59708. doi: 10.1371/journal.pone.0059708. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527257 Free PMC article. - Suppressive Effects of EGCG on Cervical Cancer.
Wang YQ, Lu JL, Liang YR, Li QS. Wang YQ, et al. Molecules. 2018 Sep 12;23(9):2334. doi: 10.3390/molecules23092334. Molecules. 2018. PMID: 30213130 Free PMC article. Review. - Targeting the MET receptor tyrosine kinase in non-small cell lung cancer: emerging role of tivantinib.
Agwa ES, Ma PC. Agwa ES, et al. Cancer Manag Res. 2014 Oct 4;6:397-404. doi: 10.2147/CMAR.S37345. eCollection 2014. Cancer Manag Res. 2014. PMID: 25328417 Free PMC article. Review.
Cited by
- A derivative of epigallocatechin-3-gallate induces apoptosis via SHP-1-mediated suppression of BCR-ABL and STAT3 signalling in chronic myelogenous leukaemia.
Jung JH, Yun M, Choo EJ, Kim SH, Jeong MS, Jung DB, Lee H, Kim EO, Kato N, Kim B, Srivastava SK, Kaihatsu K, Kim SH. Jung JH, et al. Br J Pharmacol. 2015 Jul;172(14):3565-78. doi: 10.1111/bph.13146. Epub 2015 Jun 4. Br J Pharmacol. 2015. PMID: 25825203 Free PMC article. - Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds.
Fujiki H, Sueoka E, Watanabe T, Suganuma M. Fujiki H, et al. J Cancer Res Clin Oncol. 2015 Sep;141(9):1511-22. doi: 10.1007/s00432-014-1899-5. Epub 2014 Dec 28. J Cancer Res Clin Oncol. 2015. PMID: 25544670 Review. - Targeting epidermal growth factor receptors and downstream signaling pathways in cancer by phytochemicals.
Kadioglu O, Cao J, Saeed ME, Greten HJ, Efferth T. Kadioglu O, et al. Target Oncol. 2015 Sep;10(3):337-53. doi: 10.1007/s11523-014-0339-4. Epub 2014 Nov 21. Target Oncol. 2015. PMID: 25410594 Review. - Improving In Vivo Efficacy of Bioactive Molecules: An Overview of Potentially Antitumor Phytochemicals and Currently Available Lipid-Based Delivery Systems.
Mouhid L, Corzo-Martínez M, Torres C, Vázquez L, Reglero G, Fornari T, Ramírez de Molina A. Mouhid L, et al. J Oncol. 2017;2017:7351976. doi: 10.1155/2017/7351976. Epub 2017 May 7. J Oncol. 2017. PMID: 28555156 Free PMC article. Review. - Bax/Bcl-2 Cascade Is Regulated by the EGFR Pathway: Therapeutic Targeting of Non-Small Cell Lung Cancer.
Alam M, Alam S, Shamsi A, Adnan M, Elasbali AM, Al-Soud WA, Alreshidi M, Hawsawi YM, Tippana A, Pasupuleti VR, Hassan MI. Alam M, et al. Front Oncol. 2022 Mar 25;12:869672. doi: 10.3389/fonc.2022.869672. eCollection 2022. Front Oncol. 2022. PMID: 35402265 Free PMC article. Review.
References
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. - PubMed
- Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. - PubMed
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. - PubMed
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous