Poly-(ADP-ribose) polymerase-1 is necessary for long-term facilitation in Aplysia - PubMed (original) (raw)
Poly-(ADP-ribose) polymerase-1 is necessary for long-term facilitation in Aplysia
A Iván Hernández et al. J Neurosci. 2009.
Abstract
Activity-dependent long-term synaptic plasticity requires gene expression and protein synthesis. Identifying essential genes and studying their transcriptional and translational regulation are key steps to understanding how synaptic changes become long lasting. Recently, the enzyme poly-(ADP-ribose) polymerase 1 (PARP-1) was shown to be necessary for long-term memory (LTM) in Aplysia. Since PARP-1 decondenses chromatin, we hypothesize that this enzyme regulates the expression of specific genes essential for long-term synaptic plasticity that underlies LTM. We cloned Aplysia PARP-1 (ApPARP-1) and determined that its expression in sensory neurons is necessary for serotonin (5-HT)-mediated long-term facilitation (LTF) of sensorimotor neuron synapses. PARP enzymatic activity is also required, since transient application of PARP inhibitors blocked LTF. Differential display and RNA analysis of ganglia dissected from intact animals exposed to 5-HT identified the ribosomal RNA genes as PARP-dependent effector genes. The increase in the expression of rRNAs is long lasting and dynamic. Pulse-labeling RNA studies showed a PARP-dependent increase in rRNAs but not in the total RNA 24 h after 5-HT treatment. Moreover, the expression of both the AprpL27a (Aplysia ribosomal protein L27a) and the ApE2N (Aplysia ubiquitin-conjugating enzyme E2N) mRNAs also increased after 5-HT. Thus, our results suggest that 5-HT, in part by regulating PARP-1 activity, alters the expression of transcripts required for the synthesis of new ribosomes necessary for LTF.
Figures
Figure 1.
Characterization of ApPARP-1 sequence. The N-terminal sequence contains the conserved zinc fingers (white letters in black background; subdomain A or amino acids 19–55 and amino acids 115–153) followed by the nuclear localization signal (white letters in gray background; subdomain B or amino acids 192–214) and the automodification domain (underlined bold letters; subdomain D or amino acids 360–499). Within the catalytic domain (bold letters in gray background or amino acids 500–985) the “PARP signature” is shown (bold letters underscored in gray background; subdomain F or amino acids 832–881).
Figure 2.
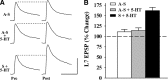
_Ap_PARP-1 expression is necessary for LTF. A, EPSPs were recorded in L7 before (Pre) and 24 h after (Post) 5 applications of 5-HT. ApPARP-1 antisense (A-S) blocked the increase in amplitude when injected into the sensory neuron 12 h before 5-HT treatment (A-S + 5-HT). In contrast, the reverse sequence of the antisense (S) did not affect LTF (S + 5-HT). In addition, injections of A-S did not affect baseline synaptic transmission (A-S). Vertical bar, 20 mV; horizontal bar, 25 ms. B, Ap-PARP-1 antisense blocked LTF produced by 5-HT. The height of each bar is the percentage change + SEM in the amplitude of the EPSP 24 h after the indicated treatments. The dashed line at 100% indicates no change with time. ANOVA indicated a significant effect of treatment (df = 2, 15; F = 17.285; p < 0.001). Individual comparisons (Scheffe F test) indicated that 5-HT applied after injection of ApPARP-1 antisense (gray bar) significantly reduced LTF compared with 5-HT applied after injection of sense oligonucleotide (black bar) (F = 12.263, p < 0.01). Injection of antisense oligonucleotide alone (A-S) did not affect synaptic baseline, and the change in EPSP was not significantly different from the change produced by 5-HT applied after injection of the antisense.
Figure 3.
Application of PARP inhibitors during 5-HT treatment blocks 24 h LTF. PARP inhibitor 3AB (A) or PJ34 (B) blocks 24 h LTF when the drug is applied during 5-HT applications. A, B, Left, EPSPs were recorded in L7 before (Pre) and 24 h after (Post) 5-HT. Incubation with either 3AB (3AB + Cont) or PJ34 (PJ34 + Cont) did not affect synaptic baseline. Applications of 5-HT produced a significant change in EPSP amplitude that was blocked when 5-HT was applied in the presence of drug (3AB + 5-HT and PJ34 + 5-HT). Vertical bar, 20 mV; horizontal bar, 25 ms. Right, The histograms indicate that blocking PARP activity with either 3AB or PJ34 blocked LTF at 24 h. The height of each bar is the average change + SEM in the EPSPs 24 h after the indicated treatments. The dashed line at 100% indicates no change in EPSP. ANOVA indicated an effect of treatment for both drugs (A: df = 2, 18; F = 13.872; p < 0.001; and B: df = 2, 13; F = 11.9; p < 0.002). Individual comparisons indicate that 3AB or PJ34 blocked LTF; 3AB + Cont was not significantly different from 3AB + 5-HT and PJ34 + Cont was not significantly different from PJ34 + 5-HT. In contrast, 5-HT produced significant increases in the EPSP compared with 3AB + Cont (F = 11.372, p < 0.01), 3AB + 5-HT (F = 9.336, p < 0.01), PJ34 + Cont (F = 8.316, p < 0.01), and PJ34 + 5-HT (F = 9.737, p < 0.01).
Figure 4.
PARP inhibitor 3AB applied after 5-HT blocks LTF. PARP inhibitor 3AB (100 μ
m
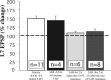
) blocks 24 h LTF when applied for 2 h immediately after (light gray bar) or 2 h after (dark gray bar) 5-HT. Exposure to vehicle (white bar) or 3AB (black bar) before 5-HT did not reduce LTF produced by 5-HT after drug washout. ANOVA indicated a significant effect of treatment (df = 3, 25; F = 16.52; p < 0.001). Individual comparisons indicated that vehicle and 3AB washout before 5-HT were not significantly different from each other. Both of these groups were significantly greater than the changes in EPSP produced when 3AB was applied immediately after 5-HT (F = 9.156, p < 0.01 and F = 3.306, p < 0.05) or 2 h after the end of 5-HT (F = 12.262, p < 0.01 and F = 4.268, p < 0.04).
Figure 5.
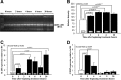
3AB blocks the upregulation of rRNA by 5-HT but not the upregulation of the immediate early gene ApC/EBP. All animals were injected 10 min before treatment with either vehicle (ASW) or 10 m
m
3AB (1 ml/90 g of body mass) and were killed after a 2 h exposure to either 5-HT or ASW. A, Five percent PAGE shows a 275 bp band (arrow) that was upregulated when animals were treated with 5-HT (compare control group in lanes 2–4 with 5-HT group in lanes 8–10). This upregulation was blocked when the PARP inhibitor 3AB was injected before 5-HT (lanes 5–7). B, One microgram of RNA from total ganglia from each animal was loaded in a 1% agarose–formaldehyde–MOPS gel. Invertebrate 28S and 18S run as a single band (top band, marked by arrow). C, Quantification of total 18S plus 28S rRNA from experiment in B. Untreated controls (white bar) expressed significantly lower levels of rRNA when compared with animals injected with vehicle before 5-HT (black bar) (LSD test, p = 0.005). The levels of rRNA expression in ganglia from animals injected with 10 m
m
3AB before 5-HT also differed significantly from animals treated with 5-HT (LSD test, p = 0.020) and were not significantly different from controls. D, Untreated controls (white bar) expressed significantly lower levels of ApC/EBP when compared with animals injected with vehicle before 5-HT (black bar) (Mann–Whitney U test, p < 0.001) or animals injected with 3AB before 5-HT (gray bar) (Mann–Whitney U test, p < 0.001). No significant difference was found between 5-HT and 5-HT + 3AB groups.
Figure 6.
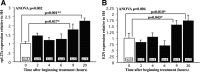
Dynamic regulation by 5-HT of the expression of rRNAs (18S plus 28S) and its unedited precursor (ht 28S) up to 20 h. A–C, 5-HT produced a long-lasting effect on rRNA expression, total rRNA (A, B), and its precursor ht 28S (C). D, As a time course control, we used the relative levels of expression of the immediate early gene ApC/EBP. Animals treated with 5-HT (black bars) were killed at different times after 2 h exposure to 5-HT (except for the group treated for 1 h in C). The control group was treated for 2 h in ASW (white bars). A, One milligram of total RNA from total ganglia from each animal was loaded in a 1% RNA gel. 5-HT produced a persistent increase in the intensity of the 18S and 28S ribosomal RNA band (arrow). B, Quantification of the RNA gel in A shows significant differences between untreated animals (0 h) and all treated groups (p values = LSD). C, Quantification of ht 28S by real-time PCR shows increases at 1, 4, 9, and 20 h after the onset of 5-HT compared with controls. D, Increase in the expression of the immediate early gene ApC/EBP quantified by real-time PCR confirms early upregulation followed by a return to basal levels at later times.
Figure 7.
Expression of rpL27a and ubiquitin-conjugating enzyme E2N mRNA expression is regulated after 5-HT. A, Time course for changes in rpL27 expression is affected by 5-HT (black). Expression of rpL27a increased significantly at later times (9 and 20 h) relative to controls. B, Time course for changes in E2N expression is affected by 5-HT (black). Expression of E2N shows a pattern of upregulation after 5-HT as that seen for rpL27a. There is a significant difference between control animals and 5-HT-treated animals at 9 and 20 h.
Similar articles
- Nucleolar integrity is required for the maintenance of long-term synaptic plasticity.
Allen KD, Gourov AV, Harte C, Gao P, Lee C, Sylvain D, Splett JM, Oxberry WC, van de Nes PS, Troy-Regier MJ, Wolk J, Alarcon JM, Hernández AI. Allen KD, et al. PLoS One. 2014 Aug 4;9(8):e104364. doi: 10.1371/journal.pone.0104364. eCollection 2014. PLoS One. 2014. PMID: 25089620 Free PMC article. - Persistent long-term synaptic plasticity requires activation of a new signaling pathway by additional stimuli.
Hu JY, Baussi O, Levine A, Chen Y, Schacher S. Hu JY, et al. J Neurosci. 2011 Jun 15;31(24):8841-50. doi: 10.1523/JNEUROSCI.1358-11.2011. J Neurosci. 2011. PMID: 21677168 Free PMC article. - Persistent Associative Plasticity at an Identified Synapse Underlying Classical Conditioning Becomes Labile with Short-Term Homosynaptic Activation.
Hu J, Schacher S. Hu J, et al. J Neurosci. 2015 Dec 9;35(49):16159-70. doi: 10.1523/JNEUROSCI.2034-15.2015. J Neurosci. 2015. PMID: 26658867 Free PMC article. - Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.
Glanzman DL. Glanzman DL. J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review. - Transcriptional regulation of long-term memory in the marine snail Aplysia.
Lee YS, Bailey CH, Kandel ER, Kaang BK. Lee YS, et al. Mol Brain. 2008 Jun 17;1:3. doi: 10.1186/1756-6606-1-3. Mol Brain. 2008. PMID: 18803855 Free PMC article. Review.
Cited by
- Epigenetic treatments for cognitive impairments.
Day JJ, Sweatt JD. Day JJ, et al. Neuropsychopharmacology. 2012 Jan;37(1):247-60. doi: 10.1038/npp.2011.85. Epub 2011 May 18. Neuropsychopharmacology. 2012. PMID: 21593731 Free PMC article. Review. - A PARP1-ERK2 synergism is required for the induction of LTP.
Visochek L, Grigoryan G, Kalal A, Milshtein-Parush H, Gazit N, Slutsky I, Yeheskel A, Shainberg A, Castiel A, Seger R, Langelier MF, Dantzer F, Pascal JM, Segal M, Cohen-Armon M. Visochek L, et al. Sci Rep. 2016 Apr 28;6:24950. doi: 10.1038/srep24950. Sci Rep. 2016. PMID: 27121568 Free PMC article. - Nucleolar integrity is required for the maintenance of long-term synaptic plasticity.
Allen KD, Gourov AV, Harte C, Gao P, Lee C, Sylvain D, Splett JM, Oxberry WC, van de Nes PS, Troy-Regier MJ, Wolk J, Alarcon JM, Hernández AI. Allen KD, et al. PLoS One. 2014 Aug 4;9(8):e104364. doi: 10.1371/journal.pone.0104364. eCollection 2014. PLoS One. 2014. PMID: 25089620 Free PMC article. - PARP-1 is required for retrieval of cocaine-associated memory by binding to the promoter of a novel gene encoding a putative transposase inhibitor.
Lax E, Friedman A, Massart R, Barnea R, Abraham L, Cheishvili D, Zada M, Ahdoot H, Bareli T, Warhaftig G, Visochek L, Suderman M, Cohen-Armon M, Szyf M, Yadid G. Lax E, et al. Mol Psychiatry. 2017 Apr;22(4):570-579. doi: 10.1038/mp.2016.119. Epub 2016 Sep 6. Mol Psychiatry. 2017. PMID: 27595592 - PARP-1 deletion promotes subventricular zone neural stem cells toward a glial fate.
Plane JM, Grossenbacher SK, Deng W. Plane JM, et al. J Neurosci Res. 2012 Aug;90(8):1489-506. doi: 10.1002/jnr.23040. Epub 2012 Mar 19. J Neurosci Res. 2012. PMID: 22431411 Free PMC article.
References
- Aalfs JD, Kingston RE. What does ‘chromatin remodeling’ mean? Trends Biochem Sci. 2000;25:548–555. - PubMed
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. - PubMed
- Bartsch D, Ghirardi M, Casadio A, Giustetto M, Karl KA, Zhu H, Kandel ER. Enhancement of memory-related long-term facilitation by ApAF, a novel transcription factor that acts downstream from both CREB1 and CREB2. Cell. 2000;103:595–608. - PubMed
- Barzilai A, Kennedy TE, Sweatt JD, Kandel ER. 5-HT modulates protein synthesis and the expression of specific proteins during long-term facilitation in Aplysia sensory neurons. Neuron. 1989;2:1577–1586. - PubMed
- Bergold PJ, Beushausen SA, Sacktor TC, Cheley S, Bayley H, Schwartz JH. A regulatory subunit of the cAMP-dependent protein kinase down-regulated in Aplysia sensory neurons during long-term sensitization. Neuron. 1992;8:387–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH48850/MH/NIMH NIH HHS/United States
- R01 MH060387/MH/NIMH NIH HHS/United States
- R01 MH048850/MH/NIMH NIH HHS/United States
- RR-10294/RR/NCRR NIH HHS/United States
- MH60387/MH/NIMH NIH HHS/United States
- P40 RR010294/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous