Thiamine-responsive megaloblastic anemia: identification of novel compound heterozygotes and mutation update - PubMed (original) (raw)
. 2009 Dec;155(6):888-892.e1.
doi: 10.1016/j.jpeds.2009.06.017. Epub 2009 Jul 29.
Inderneel Sahai, Jill F Falcone, Judy Fleming, Adam Bagg, Caterina Borgna-Pignati, Robin Casey, Luca Fabris, Elizabeth Hexner, Lulu Mathews, Maria Leticia Ribeiro, Klaas J Wierenga, Ellis J Neufeld
Affiliations
- PMID: 19643445
- PMCID: PMC2858590
- DOI: 10.1016/j.jpeds.2009.06.017
Thiamine-responsive megaloblastic anemia: identification of novel compound heterozygotes and mutation update
Anke K Bergmann et al. J Pediatr. 2009 Dec.
Abstract
Objective: To determine causative mutations and clinical status of 7 previously unreported kindreds with TRMA syndrome, (thiamine-responsive megaloblastic anemia, online Mendelian inheritance in man, no. 249270), a recessive disorder of thiamine transporter Slc19A2.
Study design: Genomic DNA was purified from blood, and SLC19A2 mutations were characterized by sequencing polymerase chain reaction-amplified coding regions and intron-exon boundaries of all probands. Compound heterozygotes were further analyzed by sequencing parents, or cloning patient genomic DNA, to ascertain that mutations were in trans.
Results: We detected 9 novel SLC19A2 mutations. Of these, 5 were missense, 3 were nonsense, and 1 was insertion. Five patients from 4 kindreds were compound heterozygotes, a finding not reported previously for this disorder, which has mostly been found in consanguineous kindreds.
Conclusion: SLC19A2 mutation sites in TRMA are heterogeneous; with no regional "hot spots." TRMA can be caused by heterozygous compound mutations; in these cases, the disorder is found in outbred populations. To the extent that heterozygous patients were ascertained at older ages, a plausible explanation is that if one or more allele(s) is not null, partial function might be preserved. Phenotypic variability may lead to underdiagnosis or diagnostic delay, as the average time between the onset of symptoms and diagnosis was 8 years in this cohort.
Figures
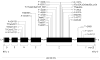
Figure 1. Known Mutations in SLC19A2 Leading to TRMA
All 28 reported mutations are presented according to their location in the thiamine transporter gene SLC19A2. Squares represent the number of unique kindreds with such mutation.
Similar articles
- Thiamine responsive megaloblastic anemia: a novel SLC19A2 compound heterozygous mutation in two siblings.
Mozzillo E, Melis D, Falco M, Fattorusso V, Taurisano R, Flanagan SE, Ellard S, Franzese A. Mozzillo E, et al. Pediatr Diabetes. 2013 Aug;14(5):384-7. doi: 10.1111/j.1399-5448.2012.00921.x. Epub 2013 Jan 4. Pediatr Diabetes. 2013. PMID: 23289844 - First 2 cases with thiamine-responsive megaloblastic anemia in the Czech Republic, a rare form of monogenic diabetes mellitus: a novel mutation in the thiamine transporter SLC19A2 gene-intron 1 mutation c.204+2T>G.
Pomahačová R, Zamboryová J, Sýkora J, Paterová P, Fiklík K, Votava T, Černá Z, Jehlička P, Lád V, Šubrt I, Dort J, Dortová E. Pomahačová R, et al. Pediatr Diabetes. 2017 Dec;18(8):844-847. doi: 10.1111/pedi.12479. Epub 2016 Dec 22. Pediatr Diabetes. 2017. PMID: 28004468 - TRMA syndrome with a severe phenotype, cerebral infarction, and novel compound heterozygous SLC19A2 mutation: a case report.
Li X, Cheng Q, Ding Y, Li Q, Yao R, Wang J, Wang X. Li X, et al. BMC Pediatr. 2019 Jul 11;19(1):233. doi: 10.1186/s12887-019-1608-2. BMC Pediatr. 2019. PMID: 31296181 Free PMC article. - Recessive SLC19A2 mutations are a cause of neonatal diabetes mellitus in thiamine-responsive megaloblastic anaemia.
Shaw-Smith C, Flanagan SE, Patch AM, Grulich-Henn J, Habeb AM, Hussain K, Pomahacova R, Matyka K, Abdullah M, Hattersley AT, Ellard S. Shaw-Smith C, et al. Pediatr Diabetes. 2012 Jun;13(4):314-21. doi: 10.1111/j.1399-5448.2012.00855.x. Epub 2012 Feb 27. Pediatr Diabetes. 2012. PMID: 22369132 Review. - Thiamine-responsive megaloblastic anemia syndrome: a disorder of high-affinity thiamine transport.
Neufeld EJ, Fleming JC, Tartaglini E, Steinkamp MP. Neufeld EJ, et al. Blood Cells Mol Dis. 2001 Jan-Feb;27(1):135-8. doi: 10.1006/bcmd.2000.0356. Blood Cells Mol Dis. 2001. PMID: 11358373 Review.
Cited by
- Management of diabetes mellitus in infants.
Karges B, Meissner T, Icks A, Kapellen T, Holl RW. Karges B, et al. Nat Rev Endocrinol. 2011 Nov 29;8(4):201-11. doi: 10.1038/nrendo.2011.204. Nat Rev Endocrinol. 2011. PMID: 22124439 Review. - Dyserythropoiesis and myelodysplasia in thiamine-responsive megaloblastic anemia syndrome.
Faraji-Goodarzi M, Tarhani F, Taee N. Faraji-Goodarzi M, et al. Clin Case Rep. 2020 Mar 6;8(6):991-994. doi: 10.1002/ccr3.2791. eCollection 2020 Jun. Clin Case Rep. 2020. PMID: 32577249 Free PMC article. - An Italian case series' description of thiamine responsive megaloblastic anemia syndrome: importance of early diagnosis and treatment.
Di Candia F, Di Iorio V, Tinto N, Bonfanti R, Iovino C, Rosanio FM, Fedi L, Iafusco F, Arrigoni F, Malesci R, Simonelli F, Rigamonti A, Franzese A, Mozzillo E. Di Candia F, et al. Ital J Pediatr. 2023 Nov 30;49(1):158. doi: 10.1186/s13052-023-01553-1. Ital J Pediatr. 2023. PMID: 38037112 Free PMC article. - Thiamine responsive megaloblastic anemia syndrome associated with patent ductus arteriosus: First case report from Kashmir Valley of the Indian subcontinent.
Ganie MA, Ali I, Ahangar AG, Wani MM, Ahmed S, Bhat MA, Seth S, Mudasir S. Ganie MA, et al. Indian J Endocrinol Metab. 2012 Jul;16(4):646-50. doi: 10.4103/2230-8210.98033. Indian J Endocrinol Metab. 2012. PMID: 22837935 Free PMC article. - Stroke as an Initial Manifestation of Thiamine-Responsive Megaloblastic Anemia.
Madaan P, Jauhari P, Michael SN, Sinha A, Chakrabarty B, Gulati S. Madaan P, et al. Ann Indian Acad Neurol. 2020 Jan-Feb;23(1):136-138. doi: 10.4103/aian.AIAN_166_19. Ann Indian Acad Neurol. 2020. PMID: 32055141 Free PMC article. No abstract available.
References
- Porter FS, Rogers LE, Sidbury JB., Jr Thiamine-responsive megaloblastic anemia. J Pediatr. 1969;74:494–504. - PubMed
- Fleming JC, Tartaglini E, Steinkamp MP, Schorderet DF, Cohen N, Neufeld EJ. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet. 1999;22:305–8. - PubMed
- Raz T, Labay V, Baron D, Szargel R, Anbinder Y, Barrett T, et al. The spectrum of mutations, including four novel ones, in the thiamine-responsive megaloblastic anemia gene SLC19A2 of eight families. Hum Mutat. 2000;16:37–42. - PubMed
- Strom TM, Hortnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet. 1998;7:2021–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical