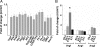Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice - PubMed (original) (raw)
Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice
Jian Xie et al. Am J Pathol. 2009 Sep.
Abstract
WNK1 [with-no-lysine (K)-1] is a ubiquitous serine/threonine kinase with a unique placement of the catalytic lysine residue. Increased WNK1 expression levels in humans causes a hypertension-hyperkalemia syndrome by altering renal Na(+) and K(+) transport. The function of WNK1 outside of the kidney remains elusive. In this study, we report that Wnk1 ablation causes cardiovascular developmental defects. The developing heart of null mutant embryos has smaller chambers and reduced myocardial trabeculation at E10.5. Yolk sac vessels in the E10.5 null mutant fail to remodel into a network of large and small vessels, and embryonic vessels show defective angiogenesis that involves both arteries and veins. The arterial marker neuropilin-1 and venous marker EphB4 are ectopically expressed in mutant veins and arteries, respectively. However, the orphan nuclear receptor COUP-TFII as well as the Notch signaling pathway, which are known to be critical for angiogenesis and artery-vein specification, are not significantly altered in Wnk1(-/-) mutants. Conditional deletion of Wnk1 in endothelial cells phenotypically copies defects caused by global Wnk1 ablation. Moreover, endothelial-specific expression of a Wnk1 transgene rescues cardiovascular developmental defects in Wnk1(-/-) mice. These findings identify a novel function of WNK1 in endothelial cells that is critical for angiogenesis and heart development, raising the possibility for a role of endothelial WNK1 in the control of blood pressure and postnatal angiogenesis and cardiac growth.
Figures
Figure 1
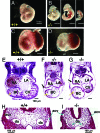
Developmental defects of _Wnk1_−/− mutant. A–D: Yolk sac, placenta, and embryo of E10.5 wild-type (A, C) and _Wnk1_−/− mutants (B, D). White arrows and arrowheads in D indicate pericardial edema and hemorrhage in some mutant embryos, respectively. E–I: H&E staining of transverse sections of E10.5 wild-type (E, H) and mutant embryos (F, G, I). RA, right atrium; LA, left atrium; BC, bulbus cordis; CV, common ventricle; da, dorsal aorta; acv, anterior cardinal vein. Yellow and black arrows in E–G indicate ventricular trabeculation and dilatation of pericardial sac in mutants, respectively. Arrows in H and I indicate dorsal aorta (da) or anterior cardinal vein (acv).
Figure 2
Immunostaining with anti-PECAM1 antibody shows vascular endothelial defects. A–E: Whole-mount staining of E10.5 wild-type and mutant embryos. White and yellow arrows (B, C) indicate internal carotid artery and head vein, respectively. Yellow arrows in D and E indicate position and density of small lateral branches sprouting between intersomitic vessels. F–I: PECAM1 staining of wild-type (F, G) and mutant yolk sacs (H, I) shown at ×20 (F, H) or ×60 magnification (G, I).
Figure 3
Placental defects of _Wnk1_−/− mutant. A and B: H&E-stained sections of E10.5 placenta (A, ×25; B, ×100 magnification) show that the labyrinthine layer (La, where embryonic vessels are in contact with maternal circulation) is thinner in mutant placenta. In the wild-type placenta, an intricate labyrinthine vascular network is evident with deep penetration of embryonic vessels and juxtaposition of maternal sinuses. In mutant placenta, the penetration of embryonic vessels is relatively superficial and there is much less contact between embryonic and maternal vessels. Arrows in B indicate embryonic erythrocytes (immature, larger nuclei) in embryonic vessels. Arrowheads indicate maternal erythrocytes in maternal sinuses. Al, allantois; La, labyrinth; Sp, spongiotrophoblast; Gc, giant cells; De, maternal decidua.
Figure 4
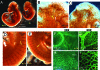
Aberrant expression of arterial and venous markers in mutant arteries and veins. A–D: The arterial marker neuropilin-1 (NP1, green) and venous marker ephrin receptor B4 (EphB4, yellow) are expressed in the dorsal aorta (A) and cardinal vein (V) of wild-type embryos, respectively (A and B). In an E9.5 mutant (C), NPI, however, is expressed in veins (V) as well as aortae (A). Similarly, EphB4 is expressed in both aorta (A) and vein (V) of mutant embryo (D). E: H&E-stained transverse sections at the level similar to that in A–D. nt, neural tube; fg, foregut; da, dorsal aorta; acv, anterior cardinal vein. F–H: Double staining of NP1 (F, red) and PECAM1 (G, green) in mutant vessels. H is an overlaid image of F and G. I: A H&E-stained transverse section at the level similar to that in F–H.
Figure 5
Expression of angiogenesis-related genes in _Wnk1_−/− embryos relative to wild-type at embryonic days 9–11. A: Expression of gene in _Wnk1_−/− embryos (KO) relative to wild-type (WT) at embryonic day 9 (E9.0). B: Relative expression of a subset of genes at embryonic days 9, 9.5, 10.5, and 11. Gene expression was analyzed by quantitative real-time PCR. Each experiment includes four to six samples for KO and WT, respectively. Results are mean ± SEM from three separate experiments. Except for VEGF at E10.5 (*P < 0.01 by unpaired two-tailed _t_-test), none of the genes analyzed are statistically significantly different between KO and WT.
Figure 6
Expression of Wnk1 in E10 embryos and yolk sacs. In situ hybridization using RNA probe against exon 6-9 of Wnk1 was performed in E10 embryos sectioned as diagrammed (A). Wnk1 is ubiquitously expressed in wild-type embryos (B). nt, neural tube; v, a branch of vein; ba, brachial arch; h, heart; g, gut; da, dorsal aorta. In the developing vessels of embryo proper (D) and yolk sac (F), strong Wnk1 signal is detected in both endothelial cells (arrow) and pericytes (arrowhead). In the developing heart (E), both endothelial lining (arrow) and trabeculated myocardium (asterisk) are positive for Wnk1. As negative controls, no signal is detected in _Wnk1_−/− embryo (C) and yolk sac (G).
Figure 7
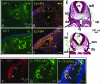
Endothelial-specific conditional knockout of Wnk1. A: Diagram for Wnk1 exon 2-floxed targeting construct and location of primers used for genotyping of targeted locus and Cre-mediated excised targeted locus. The exon 2 of Wnk1 and two ∼3-kb genomic fragments flanking exon 2 (left and right arm) were PCR-amplified and subcloned into a targeting vector such that exon 2 of Wnk1 was followed by a neo cassette, and exon 2-neo was flanked by two loxP sites. This targeting construct was used to generate Wnk1 flox mice, which were crossed with Tie2-Cre mice to produce an endothelial-specific conditional Wnk1 knockout. Forward primers cF1 and cF2 are located in the 3′ end of left arm and exon 2, respectively. Reverse primers cR1 and cR2 are located in the right arm and at the beginning of neo cassette, respectively. Sequences of primers are in Table 1. B: Genotyping of conditional knockout mice. The cF2 and cR2 primer pair identifies the targeted Wnk1 flox allele with a ∼180-bp product; while cF2 and cR1 primer pair amplifies the wild-type allele into a ∼350-bp band. Thus, PCR reaction using mixtures of cF2 forward primer and cR1 and cR2 reverse primers distinguishes among Wnk1+/+, Wnk1 flox/+, and Wnk1 flox/flox (lanes 3, 4, and 5, respectively). The cF1 and cR1 primer pair amplifies the Cre-excised Wnk1 flox/flox locus into a ∼420-bp product (lane 2), which can be distinguished from the ∼680-bp product of wild-type locus (lane 3). The much larger product of unexcised Wnk1 flox/flox locus was not amplified by PCR under the condition used (lane 1). C and D: Yolk sac of E10.5 endothelial-specific conditional Wnk1 mutant (D) and Wnk1 flox/flox but Cre-negative control (C). Arrow in C indicates the network of large and small vessels evident in the control yolk sac, which is notably absent in conditional Wnk1 mutant (D). E and F: Embryo of E10.5 endothelial-specific conditional Wnk1 mutant (F) and Wnk1 flox/flox but Cre-negative control (E). White arrow in F indicates the rim of the dilated pericardial sac in conditional Wnk1 mutants (ie, pericardial edema).
Figure 8
Detailed phenotypic features of E10.5 embryos and yolk sacs in mice of endothelial-specific deletion of Wnk1. A–D: H&E-stained transverse sections of E10.5 Wnk1 flox/flox (A, C) and Wnk1 flox/flox;Tie2-Cre (B, D) embryos. nt, neural tube; da, dorsal aorta; acv, anterior cardinal vein; RA, right atrium; LA, left atrium; BC, bulbus cordis; CV, common ventricle. The conditional knockout embryos have smaller or collapsed vessels (B), less trabeculation (yellow arrow in B, D), and dilated pericardial cavity (black arrow in D). E–J: PECAM-stained E10.5 yolk sacs (E, F) and embryos (G–J). Compared with Wnk1 flox/flox, Wnk1 flox/flox,Tie2-Cre yolk sacs have defective remodeling (E versus F). Compared with Wnk1 flox/flox embryos, Wnk1 flox/flox,Tie2-Cre embryos have atretic and disorganized branches of internal carotid artery and head veins (white and yellow arrow, respectively, in G and H), and collapsed aorta and cardinal veins (yellow arrow and yellow arrowhead, respectively, in I and J). K and L: Tracing of heart and major vessels in I and J, respectively. a, atria; aa, aortic arches; ao, aorta; cv, cardinal vein; ccv, common cardinal vein; sv, sinus venosus, v, ventricle.
Figure 9
Endothelial-specific expression of Wnk1 transgene rescues cardiovascular developmental defects and the embryonic lethality that results from global Wnk1 ablation. A: Construct for targeting full-length rat WNK1 cDNA downstream of a floxed transcriptional stopper cassette (PGK-neo tpA) to the ROSA26 locus and diagram for location of primers used for genotyping of targeted ROSA-Wnk1 (RW) allele and Cre-excised targeted allele (RWΔ). Reverse primer of RW targeted allele (R1) is located at the 5′ end of the rat WNK1 cDNA. Forward primers located upstream of the PGK-neo-tpA cassette (F1) and within the cassette (F2) distinguish the excised (active) ROSA26-Wnk1 targeted allele (RWΔ) from the unexcised (inactive) targeted allele (RW). B: Tail DNA was genotyped by PCR using mixtures of F1 and F2 forward primers and R2 reverse primer. The ∼450-bp band amplified by F1/R1 primer pair indicates presence of the excised targeted allele (RWΔ). The ∼370-bp band amplified by F2/R1 primer pair indicates presence of unexcised targeted allele (RW). PCR primers for transgenic Cre lines were as described in the Jackson Laboratories genotyping protocol. C and D: E15.5 wild-type (C) and rescued (D) embryos. E and F: Longitudinal sections of the heart of E15.5 wild-type (E) and rescued (F) embryos.
Similar articles
- WNK1 protein kinase regulates embryonic cardiovascular development through the OSR1 signaling cascade.
Xie J, Yoon J, Yang SS, Lin SH, Huang CL. Xie J, et al. J Biol Chem. 2013 Mar 22;288(12):8566-8574. doi: 10.1074/jbc.M113.451575. Epub 2013 Feb 5. J Biol Chem. 2013. PMID: 23386621 Free PMC article. - Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling.
Lai JG, Tsai SM, Tu HC, Chen WC, Kou FJ, Lu JW, Wang HD, Huang CL, Yuh CH. Lai JG, et al. PLoS One. 2014 Aug 29;9(8):e106129. doi: 10.1371/journal.pone.0106129. eCollection 2014. PLoS One. 2014. PMID: 25171174 Free PMC article. - WNK1-OSR1 Signaling Regulates Angiogenesis-Mediated Metastasis towards Developing a Combinatorial Anti-Cancer Strategy.
Hou CY, Ma CY, Lin YJ, Huang CL, Wang HD, Yuh CH. Hou CY, et al. Int J Mol Sci. 2022 Oct 11;23(20):12100. doi: 10.3390/ijms232012100. Int J Mol Sci. 2022. PMID: 36292952 Free PMC article. - WNK1 kinase signaling in metastasis and angiogenesis.
Hou CY, Ma CY, Yuh CH. Hou CY, et al. Cell Signal. 2022 Aug;96:110371. doi: 10.1016/j.cellsig.2022.110371. Epub 2022 May 29. Cell Signal. 2022. PMID: 35649473 Review. - Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.
Stalmans I. Stalmans I. Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
- Clinicopathological effects of protein phosphatase 2, regulatory subunit A, alpha mutations in gastrointestinal stromal tumors.
Toda-Ishii M, Akaike K, Suehara Y, Mukaihara K, Kubota D, Kohsaka S, Okubo T, Mitani K, Mogushi K, Takagi T, Kaneko K, Yao T, Saito T. Toda-Ishii M, et al. Mod Pathol. 2016 Nov;29(11):1424-1432. doi: 10.1038/modpathol.2016.138. Epub 2016 Jul 29. Mod Pathol. 2016. PMID: 27469332 - Vascular development in the vertebrate pancreas.
Azizoglu DB, Chong DC, Villasenor A, Magenheim J, Barry DM, Lee S, Marty-Santos L, Fu S, Dor Y, Cleaver O. Azizoglu DB, et al. Dev Biol. 2016 Dec 1;420(1):67-78. doi: 10.1016/j.ydbio.2016.10.009. Epub 2016 Oct 24. Dev Biol. 2016. PMID: 27789228 Free PMC article. - WNK pathways in cancer signaling networks.
Gallolu Kankanamalage S, Karra AS, Cobb MH. Gallolu Kankanamalage S, et al. Cell Commun Signal. 2018 Nov 3;16(1):72. doi: 10.1186/s12964-018-0287-1. Cell Commun Signal. 2018. PMID: 30390653 Free PMC article. Review. - WNK1 Kinase Stimulates Angiogenesis to Promote Tumor Growth and Metastasis.
Sie ZL, Li RY, Sampurna BP, Hsu PJ, Liu SC, Wang HD, Huang CL, Yuh CH. Sie ZL, et al. Cancers (Basel). 2020 Mar 2;12(3):575. doi: 10.3390/cancers12030575. Cancers (Basel). 2020. PMID: 32131390 Free PMC article. - Actions of the protein kinase WNK1 on endothelial cells are differentially mediated by its substrate kinases OSR1 and SPAK.
Dbouk HA, Weil LM, Perera GK, Dellinger MT, Pearson G, Brekken RA, Cobb MH. Dbouk HA, et al. Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):15999-6004. doi: 10.1073/pnas.1419057111. Epub 2014 Oct 31. Proc Natl Acad Sci U S A. 2014. PMID: 25362046 Free PMC article.
References
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. - PubMed
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. - PubMed
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. - PubMed
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous