Regulation of succinate-ubiquinone reductase and fumarate reductase activities in human complex II by phosphorylation of its flavoprotein subunit - PubMed (original) (raw)
Regulation of succinate-ubiquinone reductase and fumarate reductase activities in human complex II by phosphorylation of its flavoprotein subunit
Eriko Tomitsuka et al. Proc Jpn Acad Ser B Phys Biol Sci. 2009.
Abstract
Complex II (succinate-ubiquinone reductase; SQR) is a mitochondrial respiratory chain enzyme that is directly involved in the TCA cycle. Complex II exerts a reverse reaction, fumarate reductase (FRD) activity, in various species such as bacteria, parasitic helminths and shellfish, but the existence of FRD activity in humans has not been previously reported. Here, we describe the detection of FRD activity in human cancer cells. The activity level was low, but distinct, and it increased significantly when the cells were cultured under hypoxic and glucose-deprived conditions. Treatment with phosphatase caused the dephosphorylation of flavoprotein subunit (Fp) with a concomitant increase in SQR activity, whereas FRD activity decreased. On the other hand, treatment with protein kinase caused an increase in FRD activity and a decrease in SQR activity. These data suggest that modification of the Fp subunit regulates both the SQR and FRD activities of complex II and that the phosphorylation of Fp might be important for maintaining mitochondrial energy metabolism within the tumor microenvironment.
Figures
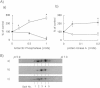
Fig. 1.
Effects of phosphatase and protein kinase on complex II. A) Effects of phosphatase and protein kinase on SQR and FRD activities in DLD-1 mitochondria. a) SQR and FRD activities after treatment with Antarctic Phosphatase in solubilized DLD-1 mitochondria. b) SQR and FRD activities after treatment with the protein kinase A catalytic subunit in solubilized DLD-1 mitochondria. The open squares show the ratio of SQR activity versus the control value, while the closed circle show the ratio of FRD activity versus the control in the absence of phosphatase/kinase. All data were expressed as the means ± S.D. of three experiments. Statistically significant differences with respect to the control value (p < 0.05) are shown by an asterisk (Student’s _t_-test). B) Separation of Fp proteins. The detection of Fp was performed using an anti-bovine heart Fp monoclonal antibody after two-dimensional gel electrophoresis in solubilized DLD-1 mitochondria. a) Untreated, b) treated with 0.5 Units Antarctic Phosphatase, and c) treated with 0.2 Units protein kinase A.
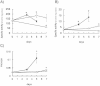
Fig. 2.
Complex II activities in mitochondria from DLD-1 under tumor-mimicking microenvironmental conditions. A) SQR activities, B) FRD activities, and C) ratios of FRD/SQR. The closed circles show the enzyme activities in solubilized mitochondria from DLD-1 cultured under hypoxic and glucose-depleted conditions. The open circles show the enzyme activities in solubilized mitochondria from DLD-1 cultured under hypoxic conditions. The open triangles show the enzyme activities in solubilized mitochondria from DLD-1 cultured under glucose-depleted conditions. All the data were expressed as the means ± S.D. of three experiments. Statistically significant differences with respect to the baseline values (p < 0.05) are shown by an asterisk (Student’s _t_-test).
Similar articles
- Comparative study and cDNA cloning of the flavoprotein subunit of mitochondrial complex II (succinate-ubiquinone oxidoreductase: fumarate reductase) from the dog heartworm, Dirofilaria immitis.
Kuramochi T, Kita K, Takamiya S, Kojima S, Hayasaki M. Kuramochi T, et al. Comp Biochem Physiol B Biochem Mol Biol. 1995 Jul;111(3):491-502. doi: 10.1016/0305-0491(95)00022-z. Comp Biochem Physiol B Biochem Mol Biol. 1995. PMID: 7613771 - Change of subunit composition of mitochondrial complex II (succinate-ubiquinone reductase/quinol-fumarate reductase) in Ascaris suum during the migration in the experimental host.
Iwata F, Shinjyo N, Amino H, Sakamoto K, Islam MK, Tsuji N, Kita K. Iwata F, et al. Parasitol Int. 2008 Mar;57(1):54-61. doi: 10.1016/j.parint.2007.08.002. Epub 2007 Aug 25. Parasitol Int. 2008. PMID: 17933581 - Complex II from a structural perspective.
Horsefield R, Iwata S, Byrne B. Horsefield R, et al. Curr Protein Pept Sci. 2004 Apr;5(2):107-18. doi: 10.2174/1389203043486847. Curr Protein Pept Sci. 2004. PMID: 15078221 - Succinate: quinone oxidoreductases: new insights from X-ray crystal structures.
Lancaster CR, Kröger A. Lancaster CR, et al. Biochim Biophys Acta. 2000 Aug 15;1459(2-3):422-31. doi: 10.1016/s0005-2728(00)00180-8. Biochim Biophys Acta. 2000. PMID: 11004459 Review. - Maturation of the respiratory complex II flavoprotein.
Sharma P, Maklashina E, Cecchini G, Iverson TM. Sharma P, et al. Curr Opin Struct Biol. 2019 Dec;59:38-46. doi: 10.1016/j.sbi.2019.01.027. Epub 2019 Mar 7. Curr Opin Struct Biol. 2019. PMID: 30851631 Free PMC article. Review.
Cited by
- Succinate dehydrogenase - Assembly, regulation and role in human disease.
Rutter J, Winge DR, Schiffman JD. Rutter J, et al. Mitochondrion. 2010 Jun;10(4):393-401. doi: 10.1016/j.mito.2010.03.001. Epub 2010 Mar 10. Mitochondrion. 2010. PMID: 20226277 Free PMC article. Review. - Mitochondrial respiration and succinate dehydrogenase are suppressed early during entrance into a hibernation bout, but membrane remodeling is only transient.
Chung D, Lloyd GP, Thomas RH, Guglielmo CG, Staples JF. Chung D, et al. J Comp Physiol B. 2011 Jul;181(5):699-711. doi: 10.1007/s00360-010-0547-x. Epub 2011 Jan 5. J Comp Physiol B. 2011. PMID: 21207037 - Alterations in cellular energy metabolism associated with the antiproliferative effects of the ATM inhibitor KU-55933 and with metformin.
Zakikhani M, Bazile M, Hashemi S, Javeshghani S, Avizonis D, St Pierre J, Pollak MN. Zakikhani M, et al. PLoS One. 2012;7(11):e49513. doi: 10.1371/journal.pone.0049513. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185347 Free PMC article. - A "Weird" Mitochondrial Fatty Acid Oxidation as a Metabolic "Secret" of Cancer.
Zhelev Z, Aoki I, Lazarova D, Vlaykova T, Higashi T, Bakalova R. Zhelev Z, et al. Oxid Med Cell Longev. 2022 Feb 8;2022:2339584. doi: 10.1155/2022/2339584. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35178152 Free PMC article. Review. - Control mechanisms in mitochondrial oxidative phosphorylation.
Hroudová J, Fišar Z. Hroudová J, et al. Neural Regen Res. 2013 Feb 5;8(4):363-75. doi: 10.3969/j.issn.1673-5374.2013.04.009. Neural Regen Res. 2013. PMID: 25206677 Free PMC article.
References
- Pawl, C., Bausch, B., Neumann, H.P.H. (2005) Mutations of the SDHB and SDHD genes. Familial Cancer 4, 49–54 - PubMed
- Muller, U., Troidl, C. and Niemann, S. (2005) SDHC mutations in hereditary paraganglioma/pheochromocytoma. Familial Cancer 4, 9–12 - PubMed
- Tomitsuka, E., Goto, Y., Taniwaki, M. and Kita, K. (2003) Direct evidence for expression of Type II flavoprotein subunit in human complex II (succinate-ubiquinone reductase). Biochem. Biophys. Res. Com. 311, 774–779 - PubMed
- Tomitsuka, E., Hirawake, H., Goto, Y., Taniwaki, M., Harada, S. and Kita, K. (2003) Direct evidence for two distinct forms of the flavoprotein subunit of human mitochondrial complex II (succinate-ubiquinone reductase). J. Biochem. 134, 191–195 - PubMed