eNOS, metabolic syndrome and cardiovascular disease - PubMed (original) (raw)
Review
eNOS, metabolic syndrome and cardiovascular disease
Paul L Huang. Trends Endocrinol Metab. 2009 Aug.
Abstract
Large epidemiologic studies have established that diabetes, hyperlipidemia and obesity all increase the risk for cardiovascular disease. However, the precise mechanisms by which these metabolic disorders increase the propensity to develop atherosclerosis are not known. Recently, the concept of the metabolic syndrome - a constellation of conditions including obesity, hypertension, hyperlipidemia and insulin resistance - has received much attention. Studies on the metabolic syndrome might enable a better understanding of the underlying biological mechanisms that lead to cardiovascular disease. This review focuses on endothelial nitric oxide synthase and summarizes evidence that a reduction in the bioavailability of endothelium-derived nitric oxide serves as a key link between metabolic disorders and cardiovascular risk.
Figures
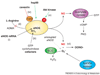
Figure 1
Regulation of eNOS activity and mechanisms of endothelial dysfunction. The bioavailability of NO produced by eNOS might be affected at multiple levels, including (i) eNOS mRNA or protein expression; (ii) availability of L-arginine, its substrate, which might be competed by ADMA; (iii) availability of its cofactors, including BH4, for which GTP cyclohydrolase catalyzes the rate-limiting step; (iv) protein–protein interactions, for example with caveolin (inhibitory; red) or hsp90 (stimulatory; green); (v) post-translational modifications, such as phosphorylation at S1177 by Akt and other kinases (stimulatory; green); and (vi) reaction of NO with superoxide to yield peroxynitrite anion. Abbreviations: ADMA, asymmetric dimethyl arginine; BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; GTP, guanosine 5′-triphosphate; hsp90, heat-shock protein 90; NO, nitric oxide; O2−, superoxide; OONO−, peroxynitrite anion; PKG, protein kinase G; SOD, superoxide dismutase.
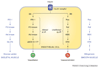
Figure 2
Insulin signaling. Insulin signaling in endothelial cells occurs after insulin binding to the insulin receptor. This causes activation of two separate and parallel pathways: (i) PI3K–Akt and (ii) Ras/Raf/MAP kinase. (i) Akt kinase phosphorylates eNOS at S1177, resulting in increased NO production and vasodilation. (ii) The MAP kinase pathway results in endothelin-1 production and vasoconstriction. In other tissues, these pathways have effects (shown in dark blue lettering). In skeletal muscle (left), the PI3K–Akt pathway results in translocation of GLUT4 and glucose uptake. In vascular smooth muscle (right), the MAP kinase pathway results in growth and mitogenesis. Abbreviations: eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; GLUT4, insulin-dependent glucose transporter 4'; MAP kinase, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3-kinase.
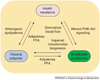
Figure 3
Relationships between insulin resistance, visceral adiposity, and endothelial dysfunction. Insulin 3 resistance can cause endothelial dysfunction through altered PI3K–Akt signaling. Endothelial dysfunction, in turn, can cause insulin resistance owing to diminished blood flow and capillary recruitment, leading to decreased substrate and insulin delivery. Endothelial dysfunction can lead to insulin resistance, as well as visceral adiposity by impaired mitochondrial biogenesis. Visceral adipose tissue can secrete adipokines and FFAs, which can cause endothelial dysfunction and peripheral insulin resistance. Finally, insulin resistance can lead to atherogenic dyslipidemia, which can contribute to visceral obesity. Abbreviations: PI3K, phosphatidylinositol-3-kinase; FFAs, free fatty acids.
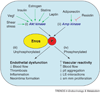
Figure 4
Role of eNOS S1177 phosphorylation in integrating effects of multiple mediators. eNOS S1177 is phosphorylated by multiple kinases, including (i) Akt kinase and (ii) AMP kinase. Shear stress, VEGF, insulin, estrogen, statins and leptin all act through Akt kinase to increase S1177 phosphorylation. Adiponectin, resistin and other metabolic signals act through AMP kinase to influence S1177 phosphorylation. (iii) The S1177 unphosphorylated form of eNOS has less enzymatic activity and is associated with lower vascular NO levels than the phosphorylated form. (iv) By contrast, S1177 phosphorylated eNOS is associated with increased vascular reactivity, higher vascular NO levels, and protective effects on vascular smooth muscle proliferation, leukocyte–endothelial interactions and platelet aggregation. Abbreviations: AMP, adenosine monophosphate; eNOS, endothelial nitric oxide synthase; LE, leukocyte–endothelial; plt, platelet; VEGF, vascular endothelial growth factor. The green arrows indicate stimulation of eNOS S1177 phosphorylation, and the red inhibitory signal indicates inhibition of S1177 phosphorylation by resistin.
Similar articles
- Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.
Tweed E, Cimova K, Craig P, Allik M, Brown D, Campbell M, Henderson D, Mayor C, Meier P, Watson N. Tweed E, et al. Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242 - Valley fever under a changing climate in the United States.
Howard MH, Sayes CM, Giesy JP, Li Y. Howard MH, et al. Environ Int. 2024 Nov;193:109066. doi: 10.1016/j.envint.2024.109066. Epub 2024 Oct 11. Environ Int. 2024. PMID: 39432997 Review.
Cited by
- Locally different endothelial nitric oxide synthase protein levels in ascending aortic aneurysms of bicuspid and tricuspid aortic valve.
Mohamed SA, Radtke A, Saraei R, Bullerdiek J, Sorani H, Nimzyk R, Karluss A, Sievers HH, Belge G. Mohamed SA, et al. Cardiol Res Pract. 2012;2012:165957. doi: 10.1155/2012/165957. Epub 2012 Jun 18. Cardiol Res Pract. 2012. PMID: 22745920 Free PMC article. - The Physiological Function of nNOS-Associated CAPON Proteins and the Roles of CAPON in Diseases.
Xie W, Xing N, Qu J, Liu D, Pang Q. Xie W, et al. Int J Mol Sci. 2023 Oct 31;24(21):15808. doi: 10.3390/ijms242115808. Int J Mol Sci. 2023. PMID: 37958792 Free PMC article. Review. - Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle.
Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, Roman MG, Adamo ML, Masters BS, Roman LJ. Hinchee-Rodriguez K, et al. Biochem Biophys Res Commun. 2013 Jun 7;435(3):501-5. doi: 10.1016/j.bbrc.2013.05.020. Epub 2013 May 14. Biochem Biophys Res Commun. 2013. PMID: 23680665 Free PMC article. - Association between atherogenic risk-modulating proteins and endothelium-dependent flow-mediated dilation in coronary artery disease patients.
Tryfonos A, Mills J, Green DJ, Wagenmakers AJM, Dawson EA, Cocks M. Tryfonos A, et al. Eur J Appl Physiol. 2023 Feb;123(2):367-380. doi: 10.1007/s00421-022-05040-z. Epub 2022 Oct 28. Eur J Appl Physiol. 2023. PMID: 36305972 Free PMC article. - Intra-abdominal fat is related to metabolic syndrome and non-alcoholic fat liver disease in obese youth.
Silveira LS, Monteiro PA, Antunes Bde M, Seraphim PM, Fernandes RA, Christofaro DG, Freitas Júnior IF. Silveira LS, et al. BMC Pediatr. 2013 Aug 7;13:115. doi: 10.1186/1471-2431-13-115. BMC Pediatr. 2013. PMID: 23919592 Free PMC article.
References
- Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. - PubMed
- Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007;92:399–404. - PubMed
- Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. - PubMed
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3421. - PubMed
- Dandona P, et al. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical