Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging - PubMed (original) (raw)
Review
Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging
Alexei Terman et al. Antioxid Redox Signal. 2010 Apr.
Abstract
It is now generally accepted that aging and eventual death of multicellular organisms is to a large extent related to macromolecular damage by mitochondrially produced reactive oxygen species, mostly affecting long-lived postmitotic cells, such as neurons and cardiac myocytes. These cells are rarely or not at all replaced during life and can be as old as the whole organism. The inherent inability of autophagy and other cellular-degradation mechanisms to remove damaged structures completely results in the progressive accumulation of garbage, including cytosolic protein aggregates, defective mitochondria, and lipofuscin, an intralysosomal indigestible material. In this review, we stress the importance of crosstalk between mitochondria and lysosomes in aging. The slow accumulation of lipofuscin within lysosomes seems to depress autophagy, resulting in reduced turnover of effective mitochondria. The latter not only are functionally deficient but also produce increased amounts of reactive oxygen species, prompting lipofuscinogenesis. Moreover, defective and enlarged mitochondria are poorly autophagocytosed and constitute a growing population of badly functioning organelles that do not fuse and exchange their contents with normal mitochondria. The progress of these changes seems to result in enhanced oxidative stress, decreased ATP production, and collapse of the cellular catabolic machinery, which eventually is incompatible with survival.
Figures
FIG. 1.
Metabolic pathways involved in the production of cellular ROS. Superoxide anion radicals (O2•−) are produced mainly in mitochondria as a result of electron leak from the electron-transport chain and to a lesser extent in the cytosol, because of the activity of one-electron transfer oxidases and the cytochrome P450 system. Superoxide rapidly dismutates spontaneously to hydrogen peroxide (H2O2), but this reaction is further increased 1,000-fold by mitochondrial and cytosolic forms of superoxide dismutase (SOD). This indicates that superoxide is a dangerous molecule, probably because of its capacity to reduce Fe(III) to Fe(II). Hydrogen peroxide, an uncharged molecule, diffuses freely within the cell. Most hydrogen peroxide is eliminated by cytosolic and mitochondrial glutathione peroxidase (GPX), as well as by catalase in peroxisomes. In the presence of redox-active iron, hydrogen peroxide is homolytically cleaved under the formation of highly reactive hydroxyl radicals (HO•; the Fenton reaction). Hydroxyl radicals can damage a variety of biomolecules, including nucleic acids, proteins, and lipids. By reacting with polyunsaturated fatty acids, they initiate a chain reaction, resulting in the formation of aldehydes that can cause additional macromolecular damage. The reaction between superoxide and nitric oxide (NO•, formed from
l
-arginine in the presence of nitric oxide synthase, NOS), produces peroxinitrite (ONOO−), which can generate a hydroxyl radical at acidic pH (e.g., in the lysosomal compartment). This possibility is provided by the fact that nitric oxide (which is uncharged and thus passes biologic membranes) can diffuse into the lysosomes, where it may react with superoxide derived from autophagocytosed mitochondria that are under degradation. Continuous arrows, transformation; dashed arrows, diffusion of substances.
FIG. 2.
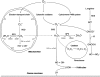
Lipofuscin accumulation and mitochondrial damage in neonatal rat cardiac myocytes. (A, B) Confocal laser scanning images (488-nm excitation) of formaldehyde-fixed cells aged 1 and 4 weeks, respectively. Lf, lipofuscin granules. (C, D) Fluorescence microscopy (blue excitation) of cardiac myocytes (aged 17 days and 3 months, respectively) vitally stained with mitochondrial tracker JC-1. Note the abundant enlarged “green” mitochondria with low membrane potential (thin arrows) and a lesser amount of slender “red” mitochondria with normal membrane potential in (D) versus (C). (E, F) The 17-day-old cells, exposed to autophagy inhibitor 3-methyladenine for 12 days, contain some enlarged mitochondria (thin arrows), as well as prominent aggregates of small mitochondria (thick arrows), many of which show a low membrane potential. Bar, 10 μm.
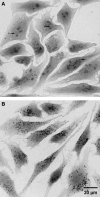
FIG. 3.
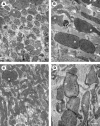
Ultrastructural mitochondrial changes associated with aging and inhibition of autophagy. (A, B) Electron microscopy images of neonatal rat cardiac myocytes cultured for 1 and 4 weeks, respectively. The aged cells contain enlarged (giant) mitochondria with irregular cristae and dense matrix. (C, D) The 17-day-old cardiac myocytes, exposed to 3-methyladenine for 12 days, accumulate numerous small, as well as some large senescent-like mitochondria (compare with Fig. 2). M, mitochondria; Lf, lipofuscin. Bar, 500 nm.
FIG. 4.
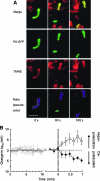
Mitochondrial fission leads to two daughter mitochondria with different membrane potentials. (A) GFP fluorescence identifies mitochondria. TMRE (tetramethylrhodamine ethyl ester) is used to calculate the membrane potential. The pseudocolor images at the bottom are used to identify the initial and daughter mitochondria. (B) Average membrane potential before (left, gray) and after fission (right, solid and empty circles denote depolarized and hyperpolarized mitochondria, respectively) are shown. Reprinted from Twig et al. (246), with permission from Macmillan Publishers Ltd. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 5.
Schematic models of mitochondrial fusion and fission. (A) Mitochondrial fusion. The outer membrane protein, Mfn, and the inner-membrane protein, OPA1, regulate mitochondrial fusion in mammalian cells. PARL cleaves the transmembrane domain of OPA1 and activates it, which may result in oligomerization and assembly in large complexes responsible for mitochondrial remodeling and cristae junction formation. (B) Mitochondrial fission. Drp-1, the key component of mitochondrial fission, is localized in the cytoplasm, but may be translocated to the mitochondrial outer membrane, triggered by an unknown signal, where it binds to other proteins and forms large circular complexes. These complexes then send signals to and from the inner membrane to coordinate the fission of both membranes, which is believed to be regulated by Rab32 and PKA. X, unidentified factors involved in fusion and fission. A complete description of the processes is given in (20).
FIG. 6.
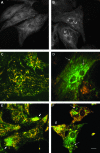
Mitochondrial fusion in mammalian cells. (A) Mitochondrial morphology is controlled by two opposing processes, fusion and fission. For mitochondrial fusion to occur, mitochondria must be in close contact. It also requires mitochondrial fusion proteins (see Fig. 5A for details), functional mitochondrial inner membrane potential, and low concentrations of GTP. (B) Fusion of two mitochondria labeled with different fluorescent proteins (e.g., GFP and DsRed2) results in the formation of a single mitochondrion with intermixed mitochondrial contents of the parent mitochondria. Giant mitochondria do not appear to fuse with normal mitochondria or with each other. (C) A fluorescence-microscopy image of a polykaryon formed by fusion of cells containing mitochondria labeled with GFP and DsRed2. Four hours after fusion, most mitochondria have fused with others and exchanged their mitochondrial matrix components, containing both fluorescent labels appearing as a yellow color in the composite image. The nuclei were counterstained with DAPI (blue). (D) A cell containing giant mitochondria labeled with mitochondria-targeted GFP were fused with other cells containing normal mitochondria labeled with DsRed2. Giant mitochondria remain single-labeled even 8 h after fusion, whereas normal mitochondria show colocalization of both fluorescent probes. (C*, D*) Enlarged sections of the images (C) and (D), respectively, with both the composite image and green and red components of the same image. Reprinted from Navratil et al. (169) with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 7.
Autophagic pathways. The cell takes up extracellular material by invagination of the plasma membrane (endocytosis), thereby forming early endosomes, which mature into acidified late endosomes. The latter receive lysosomal enzymes by fusion with secretory vesicles from the trans-Golgi network (TGN). Further maturation leads to lysosome formation. Cytosolic macromolecules may be directly engulfed by invaginations of the lysosomal membrane (microautophagy), whereas organelles (e.g., mitochondria) are being enclosed by a newly formed phagophore, resulting in the formation of an autophagosome (macroautophagy), which then fuses with either a late endosome or a lysosome (or, perhaps, with secretory vesicles from the TGN), forming an autophagolysosome. Certain proteins are delivered to lysosomes with the help of chaperones, such as Hsp73 (chaperone-mediated autophagy).
FIG. 8.
Demonstration of lysosomal iron in HeLa cells by using the sensitive cytochemical sulfide silver method. Glutaraldehyde-fixed specimens are exposed to ammonium sulfide at pH∼12 and then developed in a colloid-protected (gum arabic) solution containing silver lactate and the reducing agent hydroquinone. Tiny silver particles precipitate and gradually enlarge to a size visible by light microscopy. The process is akin to physical development of a photographic plate. After a short development time of 25 min (A), only very iron-rich lysosomes are visible (arrows). These lysosomes most probably correspond to autophagolysosomes that are engaged in the degradation of iron-containing material, such as ferrritin or mitochondrial complexes. After 40 min of development (B), a strong general lysosomal pattern is seen, reflecting the fact that most lysosomes contain some low-mass iron.
FIG. 9.
Results of intralysosomal formation of hydroxyl radicals. Hydrogen peroxide is formed normally, mainly from mitochondria. It is efficiently inactivated by the cell's antioxidative shield. Only a small portion of this oxidant manages to diffuse into lysosomes, a compartment rich in cystein and redox-active iron, the latter originating from the degradation of a variety of iron-containing proteins. Hydrogen peroxide and iron react in the Fenton reaction, yielding hydroxyl radicals. This process gives rise to intralysosomal oxidation/peroxidation with resulting damage to the lysosomal membrane and macromolecules undergoing autophagic degradation. Some oxidation products polymerize and become undegradable (lipofuscin) and accumulate in lysosomes of long-lived postmitotic cells, which do not dilute the pigment by division.
FIG. 10.
Lysosomal–mitochondrial cross-talk. Lysosomes are involved in the external as well as in the internal apoptotic pathway. In the external pathway, lysosomal destabilization can be mediated by caspase 8, either directly or indirectly, through activation of Bax or by ceramide that is converted into sphingosine, which is a lysosomotropic detergent. P53 can also destabilize lysosomes through the recently discovered LAPF protein. A variety of synthetic lysosomotropic agents (e.g., MSDH or 3-aminopropanal) can labilize lysosomes. Furthermore, the lysosomal membrane can be peroxidized and subsequently ruptured by hydroxyl radicals that originate from Fenton reaction between hydrogen peroxide and intralysosomal redox-active iron. Released lysosomal enzymes can further damage lysosomes either directly or through activation of phospholipases. The internal apoptotic pathway is activated through mitochondrial damage. This could be the result of activation of Bax or Bid, phospholipases, or lysosomal enzymes with subsequent cytochrome c release and the start of the caspase cascade, leading to apoptosis.
FIG. 11.
Mechanisms of lipofuscin formation. Superoxide (O2•−) forms mainly in mitochondria as a side product of biologic respiration. It is converted into hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Hydrogen peroxide is further homolytically split, yielding the hydroxyl radical (HO•), in the presence of ferrous iron (the Fenton reaction). Hydroxyl radicals damage surrounding macromolecules, while H2O2 diffuses throughout the cell. Oxidatively damaged macromolecules (parts of mitochondria and other cellular structures) enter lysosomes through autophagy. In the autophagolysosomes, which are rich in iron, more hydroxyl radicals form, causing oxidative damage to autophagocytosed material, resulting in its polymerization and undegradability (i.e., lipofuscin formation). Actions of lysosomal enzymes (Enz) and reactive oxygen species are indicated as dashed arrows. Black dots, oxidatively damaged macromolecules, including components of lipofuscin. Bold curved arrows, the sequence of events.
FIG. 12.
The accumulation of “waste” is a consequence of imperfect autophagy. Lysosomal enzymes are produced in the trans-Golgi network (TGN) and by secretory vesicles transported to late endosomes that acidify and maturate into lysosomes (see Fig. 7), which in turn fuse with autophagosomes (APSs). The continual fusion and fission of the lysosomal vacuoles ensures the distribution of acid hydrolases within the lysosomal compartment, including APS. In contrast to a young cell (A) that has only few lysosomes containing the undegradable age-pigment lipofuscin (Lf), senescent postmitotic cells (B) contain large numbers of Lf-containing lysosomes, to which more and more lysosomal enzymes are directed in a useless effort to degrade lipofuscin. These lysosomal enzymes are lost for useful purposes (e.g., for the degradation of newly autophagocytosed material), resulting in a delayed turnover and the accumulation of waste products. Damaged/dysfunctional mitochondria are indicated by dark shading.
FIG. 13.
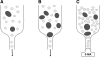
The bottleneck model of age-related accumulation of giant mitochondria. Autophagy of large mitochondria is more complicated than that of small ones. This results in progressive accumulation within long-lived postmitotic cells of enlarged (giant) mitochondria, which do not “pass the bottleneck.” (A, B) Young and senescent cells, respectively. Inhibition of autophagic sequestration with 3-methyladenine (C), which suppresses the turnover of all mitochondria independent of their size, results in the accumulation of mitochondria in quantities reflecting their turnover rates. Consequently, cells accumulate numerous small mitochondria and only few large, senescent-like mitochondria.
FIG. 14.
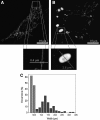
Morphology of normal and giant mitochondria in L6 rat myoblast cells. (A) Fluorescence-microscopy image of a myoblast cell containing normal mitochondria (up to 0.5 μm wide). (B) Several mitochondria are dramatically enlarged. The highlighted mitochondrion is 4.5 μm long and 2.6 μm wide. (C) The width distributions of 80 normal (light bars) and 80 giant (dark bars) mitochondria as determined by analysis of fluorescent images in SimplePCI 5.3 software. The distributions were normalized by dividing the number of hits in each bin (bin size, 0.2 μm) by 80 (i.e., the total number of mitochondria analyzed). Reprinted from Navratil et al. (169) with permission from Elsevier.
FIG. 15.
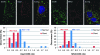
Expression of mitochondrial fusion proteins in giant mitochondria. L6 myoblasts treated with 5 m_M_ 3-MA were immunostained for cytochrome c oxidase subunit I (COXI, green) and mitochondrial fusion proteins OPA1 and Mfn2 (red). The nuclei are counterstained with DAPI in the composite images. Relative OPA1 and Mfn2 expression levels were assessed by measuring the red fluorescence signal normalized by the green COXI fluorescence in overlapping areas. Bottom plots show differences in the distributions of Opa1/COXI (left) and Mfn2/COXI (right) ratios between normal (blue) and giant (red) mitochondria. For each distribution a median value (Med) is reported. Modified from Navratil et al. (169) with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
Similar articles
- The lysosomal-mitochondrial axis theory of postmitotic aging and cell death.
Terman A, Gustafsson B, Brunk UT. Terman A, et al. Chem Biol Interact. 2006 Oct 27;163(1-2):29-37. doi: 10.1016/j.cbi.2006.04.013. Epub 2006 May 1. Chem Biol Interact. 2006. PMID: 16737690 Review. - Oxidative stress, accumulation of biological 'garbage', and aging.
Terman A, Brunk UT. Terman A, et al. Antioxid Redox Signal. 2006 Jan-Feb;8(1-2):197-204. doi: 10.1089/ars.2006.8.197. Antioxid Redox Signal. 2006. PMID: 16487053 Review. - Autophagy, organelles and ageing.
Terman A, Gustafsson B, Brunk UT. Terman A, et al. J Pathol. 2007 Jan;211(2):134-43. doi: 10.1002/path.2094. J Pathol. 2007. PMID: 17200947 Review. - Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover.
Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Terman A, et al. Ann N Y Acad Sci. 2004 Jun;1019:70-7. doi: 10.1196/annals.1297.015. Ann N Y Acad Sci. 2004. PMID: 15246997 - Catabolic insufficiency and aging.
Terman A. Terman A. Ann N Y Acad Sci. 2006 May;1067:27-36. doi: 10.1196/annals.1354.005. Ann N Y Acad Sci. 2006. PMID: 16803967 Review.
Cited by
- Protein signatures from blood plasma and urine suggest changes in vascular function and IL-12 signaling in elderly with a history of chronic diseases compared with an age-matched healthy cohort.
Yu Y, Singh H, Kwon K, Tsitrin T, Petrini J, Nelson KE, Pieper R. Yu Y, et al. Geroscience. 2021 Apr;43(2):593-606. doi: 10.1007/s11357-020-00269-y. Epub 2020 Sep 24. Geroscience. 2021. PMID: 32974878 Free PMC article. - Mitochondria and oxidative stress in heart aging.
Martín-Fernández B, Gredilla R. Martín-Fernández B, et al. Age (Dordr). 2016 Aug;38(4):225-238. doi: 10.1007/s11357-016-9933-y. Epub 2016 Jul 24. Age (Dordr). 2016. PMID: 27449187 Free PMC article. Review. - AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging.
Bujak AL, Crane JD, Lally JS, Ford RJ, Kang SJ, Rebalka IA, Green AE, Kemp BE, Hawke TJ, Schertzer JD, Steinberg GR. Bujak AL, et al. Cell Metab. 2015 Jun 2;21(6):883-90. doi: 10.1016/j.cmet.2015.05.016. Cell Metab. 2015. PMID: 26039451 Free PMC article. - The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan.
Yen K, Mehta HH, Kim SJ, Lue Y, Hoang J, Guerrero N, Port J, Bi Q, Navarrete G, Brandhorst S, Lewis KN, Wan J, Swerdloff R, Mattison JA, Buffenstein R, Breton CV, Wang C, Longo V, Atzmon G, Wallace D, Barzilai N, Cohen P. Yen K, et al. Aging (Albany NY). 2020 Jun 23;12(12):11185-11199. doi: 10.18632/aging.103534. Epub 2020 Jun 23. Aging (Albany NY). 2020. PMID: 32575074 Free PMC article. - Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly.
Kollmann TR, Levy O, Montgomery RR, Goriely S. Kollmann TR, et al. Immunity. 2012 Nov 16;37(5):771-83. doi: 10.1016/j.immuni.2012.10.014. Immunity. 2012. PMID: 23159225 Free PMC article. Review.
References
- Agarraberes FA. Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. - PubMed
- Alexander C. Votruba M. Pesch UE. Thiselton DL. Mayer S. Moore A. Rodriguez M. Kellner U. Leo-Kottler B. Auburger G. Bhattacharya SS. Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. - PubMed
- Amerik A. Antonov VK. Ostroumova NI. Rotanova TV. Chistiakova LG. [Cloning, structure and expression of the full-size lon gene in Escherichia coli coding for ATP-dependent La-proteinase] Bioorg Khim. 1990;16:869–880. - PubMed
- Annex BH. Kraus WE. Dohm GL. Williams RS. Mitochondrial biogenesis in striated muscles: rapid induction of citrate synthase mRNA by nerve stimulation. Am J Physiol. 1991;260:C266–C270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources