Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments - PubMed (original) (raw)
Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments
Nobumichi Furuta et al. Infect Immun. 2009 Oct.
Abstract
Porphyromonas gingivalis, a periodontal pathogen, secretes outer membrane vesicles (MVs) that contain major virulence factors, including major fimbriae and proteases termed gingipains, although it is not confirmed whether MVs enter host cells. In this study, we analyzed the mechanisms involved in the interactions of P. gingivalis MVs with human epithelial cells. Our results showed that MVs swiftly adhered to HeLa and immortalized human gingival epithelial cells in a fimbria-dependent manner and then entered via a lipid raft-dependent endocytic pathway. The intracellular MVs were subsequently routed to early endosome antigen 1-associated compartments and then were sorted to lysosomal compartments within 90 min, suggesting that intracellular MVs were ultimately degraded by the cellular digestive machinery. However, P. gingivalis MVs remained there for over 24 h and significantly induced acidified compartment formation after being taken up by the cellular digestive machinery. In addition, MV entry was shown to be mediated by a novel pathway for transmission of bacterial products into host cells, a Rac1-regulated pinocytic pathway that is independent of caveolin, dynamin, and clathrin. Our findings indicate that P. gingivalis MVs efficiently enter host cells via an endocytic pathway and survive within the endocyte organelles for an extended period, which provides better understanding of the role of MVs in the etiology of periodontitis.
Figures
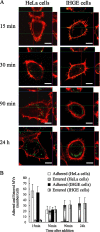
FIG. 1.
Efficient entry of P. gingivalis MVs into epithelial cells. (A) HeLa and IHGE cells were separately incubated with MVs (30 μg/ml) for 15 min and then further incubated for the indicated times. For fluorescence microscopy, the cells were processed for staining for MVs (green) and actin (Alexa Fluor 568-conjugated phalloidin red). A projection image and vertical (z) optical sections (x-z and y-z planes) are shown, although the planes have not been labeled in panel A because of spacing constraints. (For clarity, examples of labeled x-z and y-z planes can be found in Fig. 2B and 4B.) Anti-native fimbria antibodies were used to accurately trace intracellular MVs. (B) Numbers of MVs adhered to or entered into HeLa and IHGE cells at indicated times after addition. To analyze the distribution of intracellular MVs, those located inside cells more than 1 μm distant from the outermost actin filament were counted manually. Other MVs were considered to be adhered. Data represent the results of at least 120 cells from three independent experiments.
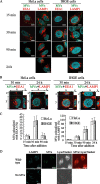
FIG. 2.
P. gingivalis MVs entered epithelial cells via the endocytic pathway. (A) Association of MVs with endocytic markers (EEA1 and LAMP1). HeLa and IHGE cells were incubated with MVs in the same manner as described in Fig. 1A. For fluorescence microscopy, the cells were processed for staining for MVs (green), EEA1 (red; left panel), and LAMP1 (red; right panel). Nuclear DNA was stained with DAPI (blue). (B) A projection image and vertical (z) optical sections (x-z and y-z planes) were used to quantitatively analyze the colocalization of MVs with endocytic markers. The cells and MVs were processed for staining as described for panel A. (C) Quantification of colocalization of MVs with endocytic markers (EEA1 and LAMP1) in HeLa and IHGE cells. The colocalization frequency is shown as the ratio of colocalized MVs to total MVs, including both intracellular and plasma membrane-associated MVs. Intracellular MVs were counted manually as described in Fig. 1B. Data shown represent the results of at least 120 cells from three independent experiments. (D) HeLa cells were incubated for 150 min after the addition of MVs and then stained for MVs (green) and with LysotrackerRed (red). Bars in each panel are 20 μm.
FIG. 3.
Immunoelectron microscopic observation of MVs entering epithelial cells. (A to E) Conventional electron microscopic images. (A) HeLa cells without addition of MVs were used as a control. (B to E) HeLa cells were incubated with MVs for 15 min, then further incubated for the indicated times. (B) HeLa cells at 90 min after addition of MVs. (D) HeLa cells at 24 h after addition of MVs. The boxed regions in panels B and D are enlarged in panels C and E, respectively. Arrows indicate intracellular MVs enwrapped with endocytic compartments. (F and G) Immunoelectron microscopic images. HeLa cells were incubated with MVs for 15 min and then further incubated for the indicated times. (F) HeLa cells at 90 min after addition of MVs. (H) HeLa cells at 24 h after addition of MVs. MVs were probed with anti-native fimbria antibodies (black). The boxed regions in panels F and H are enlarged in panels G and I, respectively. Arrowheads indicate intracellular MVs enwrapped within endocytic compartments. Bars in each panel are 500 nm.
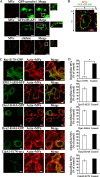
FIG. 4.
Involvement of GP1-AP1 and Rac1 in MV entry into epithelial cells. (A) Examination of colocalization of endocytic compartments carrying MVs with GPI-AP1, caveolin, and clathrin. HeLa cells were transfected with GFP-tagged caveolin-1 (green) and GPI-AP1 (GFP-GPI; green). Transfected and wild-type HeLa cells were incubated separately with MVs for 15 min and prepared for analysis at 30 min after their addition. The localization of clathrin (red) with the MVs (green) was examined using anticlathrin antibodies. (B) A projection image and vertical (z) optical sections (x-z and y-z planes) are shown. The cells and MVs were processed for staining as described in the legend to Fig. 3A. (C) Entry of MVs into DN HeLa cells. DN cells were constructed by transfection with GFP-tagged Rac1-S17N, Eps15ΔEH (clathrin DN), dynamin 1-K44A, dynamin 2-K44A, and Myc-tagged Cdc42-S17N. At 60 min after addition of MVs, the cells were processed for staining with MVs (white) and actin (red). GFP-tagged proteins are shown as green in the images. Myc-tagged Cdc42-S17N cells were stained with anti-myc (green), MVs (white), and actin (red). (D) Quantification of MVs that entered DN cells. The number of MVs that entered DN cells is shown as a percentage in comparison to wild-type HeLa cells. The data shown represent results from at least 120 cells, and each ratio (%) shows the mean value ± standard deviation from three independent experiments. Bars in each panel are 20 μm. *, P < 0.01.
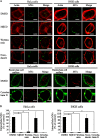
FIG. 5.
Involvement of lipid rafts, PI3K, and actin polymerization in MV entry. (A) HeLa and IHGE cells were treated with MβCD (raft-disrupting agent), wortmannin (PI3K inhibitor), cytochalasin D (an inhibitor of actin polymerization), nocodazole (microtubule assembly inhibitor), or DMSO (control) at 37°C for 30 min prior to the addition of MVs. The cells were incubated with MVs in the presence of each reagent at 37°C for 15 min and then further incubated for 120 min after addition. (B) Quantification of MVs that entered the cells in the presence of the inhibitors. The numbers of internalized MVs are shown as a percentage of those of the control (DMSO). The data shown represent results from at least 120 cells, and each ratio (%) shows the mean value ± standard deviation from three independent experiments. Bars in each panel are 20 μm. *, P < 0.01.
Similar articles
- Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment.
Furuta N, Takeuchi H, Amano A. Furuta N, et al. Infect Immun. 2009 Nov;77(11):4761-70. doi: 10.1128/IAI.00841-09. Epub 2009 Sep 8. Infect Immun. 2009. PMID: 19737899 Free PMC article. - Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway.
Takeuchi H, Furuta N, Morisaki I, Amano A. Takeuchi H, et al. Cell Microbiol. 2011 May;13(5):677-91. doi: 10.1111/j.1462-5822.2010.01564.x. Epub 2011 Jan 10. Cell Microbiol. 2011. PMID: 21155963 - A novel approach for purification and selective capture of membrane vesicles of the periodontopathic bacterium, Porphyromonas gingivalis: membrane vesicles bind to magnetic beads coated with epoxy groups in a noncovalent, species-specific manner.
Nakao R, Kikushima K, Higuchi H, Obana N, Nomura N, Bai D, Ohnishi M, Senpuku H. Nakao R, et al. PLoS One. 2014 May 15;9(5):e95137. doi: 10.1371/journal.pone.0095137. eCollection 2014. PLoS One. 2014. PMID: 24830438 Free PMC article. - Biogenesis and function of Porphyromonas gingivalis outer membrane vesicles.
Xie H. Xie H. Future Microbiol. 2015;10(9):1517-27. doi: 10.2217/fmb.15.63. Epub 2015 Sep 7. Future Microbiol. 2015. PMID: 26343879 Free PMC article. Review. - Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen.
Bostanci N, Belibasakis GN. Bostanci N, et al. FEMS Microbiol Lett. 2012 Aug;333(1):1-9. doi: 10.1111/j.1574-6968.2012.02579.x. Epub 2012 May 28. FEMS Microbiol Lett. 2012. PMID: 22530835 Review.
Cited by
- Francisella tularensis uses cholesterol and clathrin-based endocytic mechanisms to invade hepatocytes.
Law HT, Lin AE, Kim Y, Quach B, Nano FE, Guttman JA. Law HT, et al. Sci Rep. 2011;1:192. doi: 10.1038/srep00192. Epub 2011 Dec 14. Sci Rep. 2011. PMID: 22355707 Free PMC article. - Tannerella forsythia invasion in oral epithelial cells requires phosphoinositide 3-kinase activation and clathrin-mediated endocytosis.
Mishima E, Sharma A. Mishima E, et al. Microbiology (Reading). 2011 Aug;157(Pt 8):2382-2391. doi: 10.1099/mic.0.048975-0. Epub 2011 May 26. Microbiology (Reading). 2011. PMID: 21622527 Free PMC article. - Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways.
Olofsson A, Nygård Skalman L, Obi I, Lundmark R, Arnqvist A. Olofsson A, et al. mBio. 2014 May 20;5(3):e00979-14. doi: 10.1128/mBio.00979-14. mBio. 2014. PMID: 24846379 Free PMC article. - Surface Engineering of _Escherichia coli_-Derived OMVs as Promising Nano-Carriers to Target EGFR-Overexpressing Breast Cancer Cells.
Sepahdar Z, Miroliaei M, Bouzari S, Khalaj V, Salimi M. Sepahdar Z, et al. Front Pharmacol. 2021 Nov 18;12:719289. doi: 10.3389/fphar.2021.719289. eCollection 2021. Front Pharmacol. 2021. PMID: 34867325 Free PMC article. - Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis.
Śmiga M, Smalley JW, Ślęzak P, Brown JL, Siemińska K, Jenkins RE, Yates EA, Olczak T. Śmiga M, et al. Int J Mol Sci. 2021 Nov 8;22(21):12084. doi: 10.3390/ijms222112084. Int J Mol Sci. 2021. PMID: 34769513 Free PMC article.
References
- Amano, A., I. Nakagawa, N. Okahashi, and N. Hamada. 2004. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal Res. 39:136-142. - PubMed
- Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. - PubMed
- Benmerah, A., M. Bayrou, N. Cerf Bensussan, and A. Dautry Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials