A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD) - PubMed (original) (raw)
Randomized Controlled Trial
A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD)
Barnett S Meyers et al. Arch Gen Psychiatry. 2009 Aug.
Erratum in
- Arch Gen Psychiatry. 2011 Jun;68(6):626
Abstract
Context: Evidence for the efficacy of combination pharmacotherapy has been limited and without positive trials in geriatric patients with major depression (MD) with psychotic features.
Objectives: To compare remission rates of MD with psychotic features in those treated with a combination of atypical antipsychotic medication plus a serotonin reuptake inhibitor with those treated with antipsychotic monotherapy; and to compare response by age.
Design: Twelve-week, double-blind, randomized, controlled trial.
Setting: Clinical services of 4 academic sites. Patients Two hundred fifty-nine subjects with MD with psychotic features randomized by age (<60 or > or =60 years) (mean [standard deviation (SD)], 41.3 [10.8] years in 117 younger adults vs 71.7 [7.8] years in 142 geriatric participants). Intervention Target doses of 15 to 20 mg of olanzapine per day plus masked sertraline or placebo at 150 to 200 mg per day. Main Outcome Measure Remission rates of MD with psychotic features.
Results: Treatment with olanzapine/sertraline was associated with higher remission rates during the trial than olanzapine/placebo (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.12-1.47; P < .001); 41.9% of subjects who underwent combination therapy were in remission at their last assessment compared with 23.9% of subjects treated with monotherapy (chi(2)(1) = 9.53, P = .002). Combination therapy was comparably superior in both younger (OR, 1.25; 95% CI, 1.05-1.50; P = .02) and older (OR, 1.34; 95% CI, 1.09-1.66; P = .01) adults. Overall, tolerability was comparable across age groups. Both age groups had significant increases in cholesterol and triglyceride concentrations, but statistically significant increases in glucose occurred only in younger adults. Younger adults gained significantly more weight than older subjects (mean [SD], 6.5 [6.6] kg vs 3.3 [4.9] kg, P = .001).
Conclusions: Combination pharmacotherapy is efficacious for the treatment of MD with psychotic features. Future research must determine the benefits vs risks of continuing atypical antipsychotic medications beyond 12 weeks.
Trial registration: clinicaltrials.gov Identifier: NCT00056472.
Figures
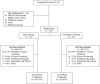
Figure 1
CONSORT CHART
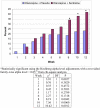
Figure 2
Remission Rates in the ITT sample of 259 Subjects Randomized To Olanzapine Plus Placebo Versus Olanzapine Plus Sertraline At Each Assessment *Statistically significant using the Hochberg alpha-level adjustments with a two-sided family-wise alpha level = 0.05 from chi-square analysis.
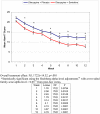
Figure 3
Ham-D Scores during the Trial in Olanzapine plus Placebo versus Olanzapine plus Sertraline Subjects Overall treatment effect: F(1,1722)=14.32, p<.001 *Statistically significant using the Hochberg alpha-level adjustments with a two-sided family-wise alpha level = 0.05 from post-hoc t-tests.
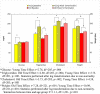
Figure 4
Metabolic Values at Baseline and Week 12 or Termination in Young Adult and Older Subjects *Glucose: Young Time Effect: t=2.76, df=205, p=.006 *Triglycerides: Old Time Effect: t=2.88. df=201, p=.004; Young Time Effect: t=3.73, df=201, p<.001. Statistics performed after log transformation due to non-normality. *Cholesterol: Old Time Effect: t=3.73, df=205, p=.002; Young Time Effect: t=4.58, df=205, p<.001 *Weight: Old Time Effect: t=7.28, df=221, p<.001; Young Time Effect: t=10.98, df=221,p<.001; Statistics performed after log transformation due to non-normality. +Interaction between time and age group: F=11.1, df=1,221, p=.001
Similar articles
- Effect of Continuing Olanzapine vs Placebo on Relapse Among Patients With Psychotic Depression in Remission: The STOP-PD II Randomized Clinical Trial.
Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Alexopoulos GS, Rudorfer MV, Marino P, Banerjee S, Pollari CD, Wu Y, Voineskos AN, Mulsant BH; STOP-PD II Study Group. Flint AJ, et al. JAMA. 2019 Aug 20;322(7):622-631. doi: 10.1001/jama.2019.10517. JAMA. 2019. PMID: 31429896 Free PMC article. Clinical Trial. - Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial.
Flint AJ, Iaboni A, Mulsant BH, Rothschild AJ, Whyte EM, Meyers BS; STOP-PD Study Group. Flint AJ, et al. Am J Geriatr Psychiatry. 2014 Apr;22(4):332-6. doi: 10.1016/j.jagp.2013.01.067. Epub 2013 May 2. Am J Geriatr Psychiatry. 2014. PMID: 23642462 Free PMC article. Clinical Trial. - Factors associated with non-completion in a double-blind randomized controlled trial of olanzapine plus sertraline versus olanzapine plus placebo for psychotic depression.
Weissman J, Flint A, Meyers B, Ghosh S, Mulsant B, Rothschild A, Whyte E; STOP-PD Study Group. Weissman J, et al. Psychiatry Res. 2012 May 30;197(3):221-6. doi: 10.1016/j.psychres.2012.02.015. Epub 2012 Mar 31. Psychiatry Res. 2012. PMID: 22464991 Free PMC article. Clinical Trial. - [Antipsychotics in bipolar disorders].
Vacheron-Trystram MN, Braitman A, Cheref S, Auffray L. Vacheron-Trystram MN, et al. Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French. - Using antipsychotic agents in older patients.
Alexopoulos GS, Streim J, Carpenter D, Docherty JP; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Alexopoulos GS, et al. J Clin Psychiatry. 2004;65 Suppl 2:5-99; discussion 100-102; quiz 103-4. J Clin Psychiatry. 2004. PMID: 14994733 Review.
Cited by
- A genetic risk score to predict treatment nonresponse in psychotic depression.
Ter Hark SE, Coenen MJH, Vos CF, Aarnoutse RE, Nolen WA, Birkenhager TK, van den Broek WW, Schellekens AFA, Verkes RJ, Janzing JGE. Ter Hark SE, et al. Transl Psychiatry. 2024 Mar 2;14(1):132. doi: 10.1038/s41398-024-02842-x. Transl Psychiatry. 2024. PMID: 38431658 Free PMC article. Clinical Trial. - Efficacy of Low-dose Olanzapine in Combination with Sertraline on Negative Symptoms and Psychosocial Functioning in Schizophrenia: A Randomized Controlled Trial.
Xiu M, Zhao L, Sun Q, Lang X. Xiu M, et al. Curr Neuropharmacol. 2024;22(8):1406-1413. doi: 10.2174/1570159X21666230913152344. Curr Neuropharmacol. 2024. PMID: 37711125 Free PMC article. Clinical Trial. - Effects of antipsychotic medication on functional connectivity in major depressive disorder with psychotic features.
Neufeld NH, Oliver LD, Mulsant BH, Alexopoulos GS, Hoptman MJ, Tani H, Marino P, Meyers BS, Rothschild AJ, Whyte EM, Bingham KS, Flint AJ, Voineskos AN. Neufeld NH, et al. Mol Psychiatry. 2023 Aug;28(8):3305-3313. doi: 10.1038/s41380-023-02118-8. Epub 2023 May 31. Mol Psychiatry. 2023. PMID: 37258617 Clinical Trial. - Effects of low-dose combined olanzapine and sertraline on negative and depressive symptoms in treatment-resistant outpatients with acute exacerbated schizophrenia.
Lang X, Zang X, Yu F, Xiu M. Lang X, et al. Front Pharmacol. 2023 Apr 21;14:1166507. doi: 10.3389/fphar.2023.1166507. eCollection 2023. Front Pharmacol. 2023. PMID: 37153770 Free PMC article. - Genomic Investigation of Remission and Relapse of Psychotic Depression Treated with Sertraline plus Olanzapine: The STOP-PD II Study.
Men X, Marshe V, Elsheikh SS, Alexopoulos GS, Marino P, Meyers BS, Mulsant BH, Rothschild AJ, Voineskos AN, Whyte EM, Kennedy JL, Flint AJ, Müller DJ. Men X, et al. Neuropsychobiology. 2023;82(3):168-178. doi: 10.1159/000529637. Epub 2023 Apr 4. Neuropsychobiology. 2023. PMID: 37015192 Free PMC article. Clinical Trial.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, DC: 2000. Text Revision.
- Johnson J, Horwath E, Weissman MM. The validity of major depression with psychotic features based on a community study. Arch Gen Psychiatry. 1991 Dec;48(12):1075–1081. - PubMed
- Ohayon MM, Schatzberg AF. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002 Nov;159(11):1855–1861. - PubMed
- Coryell W, Leon A, Winokur G, et al. Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry. 1996 Apr;153(4):483–489. - PubMed
- Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Phenomenology and prognostic significance of delusions in major depressive disorder: a 10-year prospective follow-up study. J Clin Psychiatry. 2007 Sep;68(9):1411–1417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 MH062518/MH/NIMH NIH HHS/United States
- K24 MH069430/MH/NIMH NIH HHS/United States
- U01 MH062446/MH/NIMH NIH HHS/United States
- K23 MH067710/MH/NIMH NIH HHS/United States
- MH 62518/MH/NIMH NIH HHS/United States
- MH 62446/MH/NIMH NIH HHS/United States
- MH 62565/MH/NIMH NIH HHS/United States
- MH067710/MH/NIMH NIH HHS/United States
- M01-RR024153/RR/NCRR NIH HHS/United States
- CTSC UL1RR024996/RR/NCRR NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- M01 RR000056/RR/NCRR NIH HHS/United States
- P30 MH068368/MH/NIMH NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- U01 MH062518-01A2/MH/NIMH NIH HHS/United States
- MH 62624/MH/NIMH NIH HHS/United States
- RR000056/RR/NCRR NIH HHS/United States
- MH069430/MH/NIMH NIH HHS/United States
- U01 MH062624/MH/NIMH NIH HHS/United States
- U01 MH062565/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical