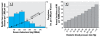Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes - PubMed (original) (raw)
Review
. 2009 Jul;117(7):1033-41.
doi: 10.1289/ehp.0800247. Epub 2009 Feb 12.
Affiliations
- PMID: 19654909
- PMCID: PMC2717126
- DOI: 10.1289/ehp.0800247
Review
Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes
Mark D Miller et al. Environ Health Perspect. 2009 Jul.
Abstract
Background: There is increasing evidence in humans and in experimental animals for a relationship between exposure to specific environmental chemicals and perturbations in levels of critically important thyroid hormones (THs). Identification and proper interpretation of these relationships are required for accurate assessment of risk to public health.
Objectives: We review the role of TH in nervous system development and specific outcomes in adults, the impact of xenobiotics on thyroid signaling, the relationship between adverse outcomes of thyroid disruption and upstream causal biomarkers, and the societal implications of perturbations in thyroid signaling by xenobiotic chemicals.
Data sources: We drew on an extensive body of epidemiologic, toxicologic, and mechanistic studies.
Data synthesis: THs are critical for normal nervous system development, and decreased maternal TH levels are associated with adverse neuropsychological development in children. In adult humans, increased thyroid-stimulating hormone is associated with increased blood pressure and poorer blood lipid profiles, both risk factors for cardiovascular disease and death. These effects of thyroid suppression are observed even within the "normal" range for the population. Environmental chemicals may affect thyroid homeostasis by a number of mechanisms, and multiple chemicals have been identified that interfere with thyroid function by each of the identified mechanisms.
Conclusions: Individuals are potentially vulnerable to adverse effects as a consequence of exposure to thyroid-disrupting chemicals. Any degree of thyroid disruption that affects TH levels on a population basis should be considered a biomarker of adverse outcomes, which may have important societal outcomes.
Keywords: children’s health; endocrine disruption; hazard identification; risk assessment; science policy; thyroid hormone; toxicologic assessments.
Figures
Figure 1
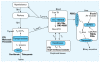
TH control pathways and sites of disruption by xenobiotic chemicals. Abbreviations: Gluc, glucose; HO-PCBs, hydroxyl-PCBs; NIS, sodium/iodide symporter; PBDE, polybrominated diphenyl ether; PTU, propylthiouracil; T4-Gluc, T4-glucuronide; TBG, thyroid-binding globulin; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone; TTR, transthyretin; UDPGT, uridine diphosphate glucuronyl-transferase. Sites or processes where xenobiotics are known or hypothesized to act as TDCs are indicated in the boxes and ovals. Xenobiotics that block, inhibit, or up -regulate these processes are shown in bold (modified from Crofton 2008).
Figure 2
Population changes in diastolic blood pressure (A) and cholesterol (B) in relation to serum TSH or free T4, respectively. (A) Diastolic blood pressure in men and women are significantly correlated with serum TSH within the normal reference range for TSH, indicating that as serum T4 declines, diastolic blood pressure increases. (B) Serum cholesterol is negatively associated with serum free T4. An increase in free T4 by 5, 10, or 15 pmol/L would reduce LDL cholesterol by 0.13, 0.53, and 0.93 mmol/L, respectively. The data are redrawn with permission from Asvold (2007b; A) and from Razvi (2007; B) (Copyrights 2007, The Endocrine Society).
Figure 3
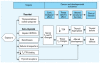
A combined mode-of-action model for the effects of TDCs on cancer and developmental outcomes. Abbreviations: TTR, transthyretin; UDPGT, uridine diphosphate glucuronyltransferase. Mixture models are needed to better predict effects of mixtures containing xenobiotics that affect multiple targets with common downstream effects (modified from Crofton and Zoeller 2005; U.S. EPA 2002).
Figure 4
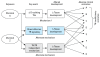
Diagnostic relationships between upstream biomarkers and adverse outcomes.
Figure 5
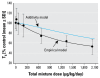
The predicted and empirical effects of a mixture of dioxins, furans, and PCBs on serum total T4 in rats. Predicted outcomes (additivity model) were generated using a single chemical-required additivity model. Empirical results (empirical model) showed a small but significant departure from dose additivity at the three highest mixture doses, whereas the remaining lower mixture doses were not significantly different than that predicted by additivity (modified from Crofton et al. 2005).
Figure 6
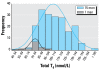
Individual versus population reference range for T4: the distribution of 12 monthly measurements for 15 men compared with one individual. The distribution width for the individual is approximately one-half that of the group [adapted from Andersen et al. (2002); copyright 2002, The Endocrine Society].
Figure 7
Individual risk and mortality associated with MI. ( A ) Individual risk and prevalence for MI associated with increased serum cholesterol levels. The number above each bar represents estimate of attributable deaths per 1,000 per 10 years. Note that individual risk increases linearly (including within the range of values considered normal) but that most deaths attributable to increased cholesterol levels occur in the lower range, because this represents a greater proportion of the population (adapted from Rose 1981; with permission from the BMJ Publishing Group). (B) Death from MI associated with increased diastolic blood pressure in males 45–74 (age-adjusted rate) (adapted from U.S. EPA 1985).
Similar articles
- Environmental chemicals targeting thyroid.
Zoeller TR. Zoeller TR. Hormones (Athens). 2010 Jan-Mar;9(1):28-40. doi: 10.14310/horm.2002.1250. Hormones (Athens). 2010. PMID: 20363719 Review. - Thyroid disrupting chemicals: mechanisms and mixtures.
Crofton KM. Crofton KM. Int J Androl. 2008 Apr;31(2):209-23. doi: 10.1111/j.1365-2605.2007.00857.x. Epub 2008 Jan 22. Int J Androl. 2008. PMID: 18217984 Review. - Thyroid disrupting chemicals and developmental neurotoxicity - New tools and approaches to evaluate hormone action.
O'Shaughnessy KL, Gilbert ME. O'Shaughnessy KL, et al. Mol Cell Endocrinol. 2020 Dec 1;518:110663. doi: 10.1016/j.mce.2019.110663. Epub 2019 Nov 21. Mol Cell Endocrinol. 2020. PMID: 31760043 Free PMC article. Review. - Transgenerational endocrine disruption: Does elemental pollution affect egg or nestling thyroid hormone levels in a wild songbird?
Ruuskanen S, Espín S, Sánchez-Virosta P, Sarraude T, Hsu BY, Pajunen P, Costa RA, Eens M, Hargitai R, Török J, Eeva T. Ruuskanen S, et al. Environ Pollut. 2019 Apr;247:725-735. doi: 10.1016/j.envpol.2019.01.088. Epub 2019 Jan 23. Environ Pollut. 2019. PMID: 30721863 - Thyroid system-disrupting chemicals: interference with thyroid hormone binding to plasma proteins and the cellular thyroid hormone signaling pathway.
Yamauchi K, Ishihara A. Yamauchi K, et al. Rev Environ Health. 2006 Oct-Dec;21(4):229-51. doi: 10.1515/reveh.2006.21.4.229. Rev Environ Health. 2006. PMID: 17243349 Review.
Cited by
- A Novel Transgenic Model to Study Thyroid Axis Activity in Early Life Stage Medaka.
Pesce E, Garde M, Rigolet M, Tindall AJ, Lemkine GF, Baumann LA, Sachs LM, Du Pasquier D. Pesce E, et al. Environ Sci Technol. 2024 Jan 9;58(1):99-109. doi: 10.1021/acs.est.3c05515. Epub 2023 Dec 20. Environ Sci Technol. 2024. PMID: 38117130 Free PMC article. - Association between thyroid function and urinary levels of 3,5,6-trichloro-2-pyridinol: data from NHANES 2007-2008.
Jain RB. Jain RB. Environ Sci Pollut Res Int. 2017 Jan;24(3):2820-2826. doi: 10.1007/s11356-016-8007-0. Epub 2016 Nov 12. Environ Sci Pollut Res Int. 2017. PMID: 27838902 - Advancing the next generation of health risk assessment.
Cote I, Anastas PT, Birnbaum LS, Clark RM, Dix DJ, Edwards SW, Preuss PW. Cote I, et al. Environ Health Perspect. 2012 Nov;120(11):1499-502. doi: 10.1289/ehp.1104870. Epub 2012 Aug 8. Environ Health Perspect. 2012. PMID: 22875311 Free PMC article. - Interactions between chemical and climate stressors: a role for mechanistic toxicology in assessing climate change risks.
Hooper MJ, Ankley GT, Cristol DA, Maryoung LA, Noyes PD, Pinkerton KE. Hooper MJ, et al. Environ Toxicol Chem. 2013 Jan;32(1):32-48. doi: 10.1002/etc.2043. Environ Toxicol Chem. 2013. PMID: 23136056 Free PMC article. - Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Thyroid Hormones in Maternal and Cord Sera: The HOME Study, Cincinnati, USA.
Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN, Sjödin A, Yolton K, Lanphear BP, Chen A. Vuong AM, et al. Environ Health Perspect. 2015 Oct;123(10):1079-85. doi: 10.1289/ehp.1408996. Epub 2015 Apr 17. Environ Health Perspect. 2015. PMID: 25893858 Free PMC article.
References
- Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13(11):1069–1078. - PubMed
- Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87(3):1068–1072. - PubMed
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T(4) in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002) Thyroid. 2007;17(12):1211–1223. - PubMed
- Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab. 2007a;92(3):841–845. - PubMed
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007b;156(2):181–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources