Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences - PubMed (original) (raw)
doi: 10.1186/1479-5876-7-69.
Abraham Czerniak, Tally Levy, Smadar Amiur, Jennifer Gallula, Imad Matouk, Rasha Abu-lail, Vladimir Sorin, Tatiana Birman, Nathan de Groot, Abraham Hochberg, Patricia Ohana
Affiliations
- PMID: 19656414
- PMCID: PMC2734756
- DOI: 10.1186/1479-5876-7-69
Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences
Aya Mizrahi et al. J Transl Med. 2009.
Abstract
Background: Ovarian cancer ascites fluid (OCAF), contains malignant cells, is usually present in women with an advanced stage disease and currently has no effective therapy. Hence, we developed a new therapy strategy to target the expression of diphtheria toxin gene under the control of H19 regulatory sequences in ovarian tumor cells. H19 RNA is present at high levels in human cancer tissues (including ovarian cancer), while existing at a nearly undetectable level in the surrounding normal tissue.
Methods: H19 gene expression was tested in cells from OCAF by the in-situ hybridization technique (ISH) using an H19 RNA probe. The therapeutic potential of the toxin vector DTA-H19 was tested in ovarian carcinoma cell lines and in a heterotopic animal model for ovarian cancer.
Results: H19 RNA was detected in 90% of patients with OCAF as determined by ISH. Intratumoral injection of DTA-H19 into ectopically developed tumors caused 40% inhibition of tumor growth.
Conclusion: These observations may be the first step towards a major breakthrough in the treatment of human OCAF, while the effect in solid tumors required further investigation. It should enable us to identify likely non-responders in advance, and to treat patients who are resistant to all known therapies, thereby avoiding treatment failure.
Figures
Figure 1
The level of H19 transcript in RNA isolated from cells of ascites fluid of different patients determined by RT-PCR or by ISH. A. H19 transcripts in the isolated ascites cells determined by ISH analysis. A positive stained cell is marked by a black arrow. B. The level of CA-125 in cells isolated from ascites fluid determined by IHC analysis (× 40 magnification). Black arrows mark the strong positive stains of cells expressing CA-125. C. The H19 transcript in RNA extracted from ascites cells determined by RT-PCR analysis. "M" 100-bp molecular weight marker. Line 1 – patient #1, Line 2 – patient # 2, Line 3 – patient # 3 and Line 4 – negative control. D. RT – PCR and ISH analysis of ascites cells from different patients. The RT-PCR results are expressed as positive (+) or negative (-). The ISH results are expressed as the number of moderate to strongly H19 positive samples. The intensity of hybridization signal was indicated as (+1) for weak, (+2) for moderate and (+3) for strong signals. The quantity of the staining was referred to (+1) up to one third of the cells, (+2) one to two thirds of the cells and (+3) more than two thirds of cells (I-indicates the intensity of the signal, Q-indicates the quantity of signal). Some samples could not be analyzed due to lack of material.
Figure 2
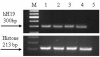
The level of the H19 transcript in human ovarian cell lines determined by RT-PCR. "M" 100-bp molecular weight marker. Line 1 – OVCAR-3, Line 2-SKOV-3, Line 3 – OV-90, Line 4 – CA-OV3, Line 5 – TOV-112D, Line 6 – ES-2 and Line 7 – negative control. The upper panel indicates the 300 bp H19 cDNA and the lower panel indicates the 300 bp histone internal control.
Figure 3
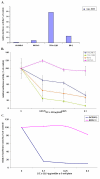
Relative luciferase activity induced by transfection of human cell lines with Luc-H19 plasmid and the reduction of luciferase activity in human ovary primary culture from patient #1 and in human ovarian carcinoma cell lines due to co-transfection with the DTA-H19 vector. A. Relative luciferase activity, in OV-CAR, SKOV-3, TOV-112D and ES-2 human cell lines induced by transfection with Luc -H19 plasmid. Each cell line was transfected with 2 μg of Luc -H19 or the LucSV40 plasmid. The values represent the luciferase activity of the H19 promoter relative to the activity of the control vector LucSV40. B. The killing potential of the DTA-H19 vector in OVCAR-3 (blue), SKOV-3 (pink), TOV-112D (green), and ES-2 (orange) was measured as a reduction of LucSV40 activity. Cells were cotransfected with 2 μg LucSV40, and the indicated concentrations of DTA-H19 or LucSV40 alone. C. The killing potential of the DTA-H19 vector in human primary culture (blue) compared with SKOV-3 (pink) was measured as a reduction of Luciferase activity. Cells were transfected with 3 μg of LucSV40 alone, or cotransfected with 3 μg LucSV40 and the indicated concentrations of DTA-H19. Transfection experiments were stopped after 48 hours and luciferase activity was assessed. The activity of the luciferase in the LucSV40 transfected cells was compared to the luciferase activity in the cotransfected cells.
Figure 4
The level of H19 transcripts in heterotopic subcutaneous tumors after injection of the ES-2 cells determined by RT-PCR. "M"100-bp molecular weight marker. Lines 1–4 – heterotopic subcutaneous tumors from different mice and Line 5 – negative control. The sizes of the PCR products are 300 bp and 213 bp for human H19 and Histone internal control respectively.
Figure 5
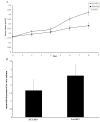
The effect of direct intratumoral injection of the DTA-H19 plasmid on subcutaneous ovarian tumor growth in nude mice. 24 mice were injected with the ES-2 cells. Starting on day 10, 12 mice received 4 injections of 25 μg of DTA-H19 plasmid and the other 12 mice received 4 injections of 25 μg of Luc-H19 plasmid complexed with PEI. Injections were given with two-day intervals. One day after the last treatment, animals were sacrificed. The tumor dimensions were measured in situ prior to the treatment with the plasmid and after sacrifice. The effect of treatments with DTA-H19 or Luc-H19 plasmids on tumor volumes (cm3) over time (days) is indicated (A), while day 0 represents the first treatment given. The mean fold increase of the final volume was compared to the initial volume in the DTA-H19 and Luc-H19 treated tumors (B).
Similar articles
- Targeting diphtheria toxin and TNF alpha expression in ovarian tumors using the H19 regulatory sequences.
Mizrahi A, Hochberg A, Amiur S, Gallula J, Matouk I, Birman T, Levy T, Ladimir S, Ohana P. Mizrahi A, et al. Int J Clin Exp Med. 2010 Sep 21;3(4):270-82. Int J Clin Exp Med. 2010. PMID: 21072261 Free PMC article. - Use of H19 regulatory sequences for targeted gene therapy in cancer.
Ohana P, Bibi O, Matouk I, Levy C, Birman T, Ariel I, Schneider T, Ayesh S, Giladi H, Laster M, de Groot N, Hochberg A. Ohana P, et al. Int J Cancer. 2002 Apr 10;98(5):645-50. doi: 10.1002/ijc.10243. Int J Cancer. 2002. PMID: 11920631 - Suicide gene strategies applied in ovarian cancer studies.
Nguyen QM, Dupré PF, Haute T, Montier T, d'Arbonneau F. Nguyen QM, et al. Cancer Gene Ther. 2023 Jun;30(6):812-821. doi: 10.1038/s41417-023-00590-6. Epub 2023 Jan 30. Cancer Gene Ther. 2023. PMID: 36717737 Review.
Cited by
- Targeting diphtheria toxin and TNF alpha expression in ovarian tumors using the H19 regulatory sequences.
Mizrahi A, Hochberg A, Amiur S, Gallula J, Matouk I, Birman T, Levy T, Ladimir S, Ohana P. Mizrahi A, et al. Int J Clin Exp Med. 2010 Sep 21;3(4):270-82. Int J Clin Exp Med. 2010. PMID: 21072261 Free PMC article. - (In)Distinctive Role of Long Non-Coding RNAs in Common and Rare Ovarian Cancers.
Sabol M, Calleja-Agius J, Di Fiore R, Suleiman S, Ozcan S, Ward MP, Ozretić P. Sabol M, et al. Cancers (Basel). 2021 Oct 9;13(20):5040. doi: 10.3390/cancers13205040. Cancers (Basel). 2021. PMID: 34680193 Free PMC article. Review. - The functions and unique features of long intergenic non-coding RNA.
Ransohoff JD, Wei Y, Khavari PA. Ransohoff JD, et al. Nat Rev Mol Cell Biol. 2018 Mar;19(3):143-157. doi: 10.1038/nrm.2017.104. Epub 2017 Nov 15. Nat Rev Mol Cell Biol. 2018. PMID: 29138516 Free PMC article. Review. - Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases.
Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM, Kumar AP, Challagundla KB, Sethi G, Gupta SC. Mishra S, et al. Cell Mol Life Sci. 2019 May;76(10):1947-1966. doi: 10.1007/s00018-019-03053-0. Epub 2019 Mar 16. Cell Mol Life Sci. 2019. PMID: 30879091 Free PMC article. Review. - Biological functions, mechanisms, and clinical significance of circular RNA in pancreatic cancer: a promising rising star.
Chen Q, Li J, Shen P, Yuan H, Yin J, Ge W, Wang W, Chen G, Yang T, Xiao B, Miao Y, Lu Z, Wu P, Jiang K. Chen Q, et al. Cell Biosci. 2022 Jun 21;12(1):97. doi: 10.1186/s13578-022-00833-3. Cell Biosci. 2022. PMID: 35729650 Free PMC article. Review.
References
- NCI National Cancer Institute http://www.cancer.gov/cancertopics/types/ovarian
- Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005;50:426–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical