Target cell type-dependent modulation of human immunodeficiency virus type 1 capsid disassembly by cyclophilin A - PubMed (original) (raw)
Target cell type-dependent modulation of human immunodeficiency virus type 1 capsid disassembly by cyclophilin A
Yuan Li et al. J Virol. 2009 Nov.
Abstract
The binding of cyclophilin A (CypA) to the human immunodeficiency virus type 1 (HIV-1) capsid protein (CA protein) is required soon after virus entry into natural target cells. In Jurkat T lymphocytes, disrupting CypA-CA interaction either by cyclosporine (Cs) treatment or by alteration (e.g., P90A) of the CA inhibits HIV-1 infection. In HeLa cells, however, treatment with Cs or Cs analogues minimally inhibits the early phase of HIV-1 infection but selects for a Cs-dependent virus with a change (A92E) in CA. To understand these phenomena, we examined the effects of the P90A and A92E changes in the HIV-1 CA protein on the stability of capsid complexes assembled in vitro and on capsid disassembly in the cytosol of virus-exposed target cells. The A92E change impaired CA-CA interactions in vitro and decreased the amount of particulate capsids in the cytosol of HeLa target cells. Reducing the binding of CypA to the A92E mutant capsid, either by Cs treatment or by an additional P90A change in the CA protein, increased the amount of particulate capsids and viral infectivity in HeLa cells. In contrast, reduction of the binding of CypA to HIV-1 capsids in Jurkat T lymphocytes resulted in a decrease in the amount of particulate capsids and infectivity. Thus, depending on the capsid and the target cell, CypA-CA binding either stabilized or destabilized the capsid, indicating that CypA modulates HIV-1 capsid disassembly. In both cell types examined, decreased stability of the capsid was associated with a decrease in the efficiency of HIV-1 infection.
Figures
FIG. 1.
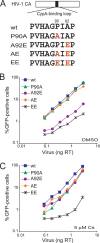
Infectivity of HIV-1 CA mutants in HeLa cells. (A) The amino acid sequences of the CypA-binding loop of the wild-type and mutant HIV-1 CA proteins is shown. (B and C) HeLa cells were infected in the presence of DMSO (B) or 5 μM Cs (C) with the indicated amounts of wild-type or mutant HIV-1-GFP. After 48 h, the percentage of GFP-positive cells was determined by FACS. The results of a single experiment typical of those obtained in three independent experiments are shown. wt, wild type.
FIG. 2.
Effect of HIV-1 CA changes on Gag processing. 293T cells were transfected with plasmids encoding the wild-type (wt) or mutant HIV-1 Gag/Pol polyproteins. Cells were harvested 48 h later, and total cellular proteins were subjected to Western blotting analysis with a monoclonal anti-HIV-1 p24 antibody (left panel). The positions of the molecular-weight-marker proteins are indicated. The virion-containing cell supernatants were centrifuged. Solubilized virion pellets were analyzed by Western blotting with a monoclonal anti-HIV-1 p24 antibody (right panel).
FIG. 3.
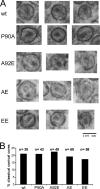
Thin-section electron microscopy of HIV-1 virions. Virions were purified from the supernatant of 293T cells transfected with plasmids encoding the wild-type or mutant HIV-1 Gag/Pol proteins and fixed with glutaraldehyde for thin-section electron microscopy analysis. (A) Examples of virions with evident conical cores are shown. (B) The indicated number (n) of virion particles was examined for each of the HIV-1 variants. The percentage of virions in which classical conical cores were evident was calculated for each variant. wt, wild type.
FIG. 4.
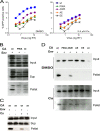
CypA incorporation into HIV-1 virions. 293T cells were cotransfected with plasmids encoding the wild-type (wt) or mutant HIV-1 Gag/Pol proteins and with a plasmid encoding a FLAG-tagged CypA (CypA-FLAG) protein. In the mock experiment, no viral or CypA-FLAG DNA was transfected. After 48 h, the transfected cells were lysed, and equal amounts of the total cellular proteins were subjected to Western blot analysis; the CypA-FLAG protein was detected with an HRP-conjugated anti-FLAG antibody (upper panel). Virions were concentrated from the supernatant of the transfected 293T cells by pelleting through a 20% sucrose cushion. Solubilized virion pellets were analyzed by Western blotting; the blots were probed with an HRP-conjugated anti-FLAG antibody (middle panel) and a monoclonal anti-HIV-1 p24 antibody (lower panel).
FIG. 5.
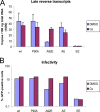
Levels of HIV-1 reverse transcripts in infected HeLa cells. (A) VSV-G-pseudotyped HIV-1-GFP viruses were normalized by RT activity and incubated with HeLa cells in the presence of DMSO or 5 μM Cs. DNA was isolated from the target cells 16 h later and the levels of viral late reverse transcription products were analyzed by real-time PCR. (B) HeLa cells were infected under the conditions described in panel A and harvested 48 h after infection. The percentage of GFP-positive cells was determined by FACS. The results of a typical experiment of two independent experiments are shown.
FIG. 6.
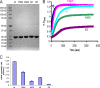
Assembly kinetics of purified HIV-1 CA proteins. (A) His-tagged wild-type or mutant HIV-1 p24 CA proteins were expressed in bacteria and purified by Ni-NTA column chromatography. Portions (20 μg) of each purified CA protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. (B) Each CA protein, at a concentration of 44 μM, was assembled in 2.5 M NaCl. The optical density at 340 nm was monitored every 10 s. (C) The initial rate of assembly for each CA protein was estimated by fitting the increase in optical density at early times (0 to 120 s) to a straight line, using a least-squares approximation. Error bars represent the standard deviations of three independent estimations. wt, wild type.
FIG. 7.
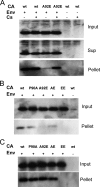
Fate of the capsid in HeLa cells infected with HIV-1 CA variants. (A) VSV-G-pseudotyped (Env+) wild-type (wt) or A92E mutant HIV-1 virions were incubated with HeLa cells in the presence or absence of 5 μM Cs. As a control, virions lacking envelope glycoproteins (Env−) were also incubated with HeLa cells under the same conditions. At 4 h after infection, the cells were washed and incubated at 37°C in fresh medium for another 12 h. After lysis of the target cells, cytoplasmic lysates were analyzed directly (“Input”) or were separated over a 50% sucrose cushion into supernatant (“Sup”) and particulate (“Pellet”) fractions. The fractions were analyzed by Western blotting, with a monoclonal anti-HIV-1 p24 antibody. The experiment was repeated twice with similar results. (B) HIV-1 CA mutants were used in the same experiment as that described in panel A, in the absence of Cs. (C) HIV-1 CA mutants were used in the same experiment as that described in panel A, in the presence of 5 μM Cs. The results of two independent experiments (Exp 1 and 2) are shown in B and C. wt, wild type.
FIG. 8.
Infectivity and fate of the capsid of HIV-1 CA variants in Jurkat T lymphocytes. (A) Jurkat cells were infected in the presence of DMSO or 2.5 μM Cs with the indicated amounts of wild-type or mutant HIV-1-GFP. The results shown are typical of those obtained in three independent experiments. (B) The fate-of-capsid assay was optimized for the study of HIV-1 infection of Jurkat cells. VSV-G-pseudotyped (Env+) wild-type or P90A HIV-1 virions were concentrated, normalized by total RT activity and then incubated with Jurkat cells. As a control, virions lacking envelope glycoproteins (Env−) were studied in parallel. Four hours after infection, the cells were washed and incubated at 37°C in fresh medium for another 12 h. After lysis of the target cells, cytoplasmic lysates were analyzed directly (“Input”) or were separated over a 50% sucrose cushion into supernatant (“Sup”) and particulate (“Pellet”) fractions. The fractions were analyzed by Western blotting, with a monoclonal anti-HIV-1 p24 antibody. (C) An experiment similar to that described in panel B was carried out, except that 5 μM Cs was added to the Jurkat cell medium as indicated. (D) HIV-1 CA variants were analyzed in an experiment similar to that described in panel B in the presence of DMSO (upper panel) or 5 μM Cs (lower panel). Note that the lower panel was exposed longer than the upper panel. The results of a single experiment are shown; the experiment was repeated twice, with comparable results. wt, wild type.
FIG. 9.
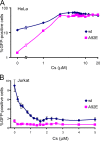
Effect of Cs titration on HIV-1 infectivity. (A) HeLa cells were infected with wild-type or A92E HIV-1-GFP in the presence of the indicated concentrations of Cs. After 48 h, the percentage of GFP-positive cells was determined by FACS. (B) Jurkat cells were used as target cells in the experiment described in panel A. Error bars represent the standard deviations obtained in at least three independent experiments. wt, wild type.
FIG. 10.
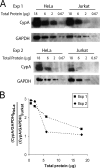
CypA expression level in HeLa and Jurkat cells. (A) Cell lysates from HeLa and Jurkat cells were normalized by total protein concentrations and subjected to Western blotting analysis. The blots were probed with an anti-CypA antibody and an HRP-conjugated anti-GAPDH antibody. The results from two independent experiments (Exp 1 and Exp. 2) are shown. (B) The ratio between CypA protein levels in HeLa cells and Jurkat cells, after normalizing to the corresponding GAPDH levels, was calculated based on quantification of protein band densities. The results from two independent experiments (Exp 1 and Exp. 2) are shown.
Similar articles
- Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells.
Hatziioannou T, Perez-Caballero D, Cowan S, Bieniasz PD. Hatziioannou T, et al. J Virol. 2005 Jan;79(1):176-83. doi: 10.1128/JVI.79.1.176-183.2005. J Virol. 2005. PMID: 15596813 Free PMC article. - Functional analysis of the secondary HIV-1 capsid binding site in the host protein cyclophilin A.
Peng W, Shi J, Márquez CL, Lau D, Walsh J, Faysal KMR, Byeon CH, Byeon IL, Aiken C, Böcking T. Peng W, et al. Retrovirology. 2019 Apr 4;16(1):10. doi: 10.1186/s12977-019-0471-4. Retrovirology. 2019. PMID: 30947724 Free PMC article. - Modulation of HIV-1 infectivity and cyclophilin A-dependence by Gag sequence and target cell type.
Matsuoka S, Dam E, Lecossier D, Clavel F, Hance AJ. Matsuoka S, et al. Retrovirology. 2009 Mar 2;6:21. doi: 10.1186/1742-4690-6-21. Retrovirology. 2009. PMID: 19254360 Free PMC article. - Emerging role of cyclophilin A in HIV-1 infection: from producer cell to the target cell nucleus.
Padron A, Prakash P, Pandhare J, Luban J, Aiken C, Balasubramaniam M, Dash C. Padron A, et al. J Virol. 2023 Nov 30;97(11):e0073223. doi: 10.1128/jvi.00732-23. Epub 2023 Oct 16. J Virol. 2023. PMID: 37843371 Free PMC article. Review. - HIV-1 capsid protein and cyclophilin a as new targets for anti-AIDS therapeutic agents.
Li J, Tang S, Hewlett I, Yang M. Li J, et al. Infect Disord Drug Targets. 2007 Sep;7(3):238-44. doi: 10.2174/187152607782110013. Infect Disord Drug Targets. 2007. PMID: 17897064 Review.
Cited by
- Cyclophilins as modulators of viral replication.
Frausto SD, Lee E, Tang H. Frausto SD, et al. Viruses. 2013 Jul 11;5(7):1684-701. doi: 10.3390/v5071684. Viruses. 2013. PMID: 23852270 Free PMC article. Review. - A Potent Postentry Restriction to Primate Lentiviruses in a Yinpterochiropteran Bat.
Morrison JH, Miller C, Bankers L, Crameri G, Wang LF, Poeschla EM. Morrison JH, et al. mBio. 2020 Sep 15;11(5):e01854-20. doi: 10.1128/mBio.01854-20. mBio. 2020. PMID: 32934084 Free PMC article. - HIV-1 capsid: the multifaceted key player in HIV-1 infection.
Campbell EM, Hope TJ. Campbell EM, et al. Nat Rev Microbiol. 2015 Aug;13(8):471-83. doi: 10.1038/nrmicro3503. Nat Rev Microbiol. 2015. PMID: 26179359 Free PMC article. Review. - HIV-1 uncoating: connection to nuclear entry and regulation by host proteins.
Ambrose Z, Aiken C. Ambrose Z, et al. Virology. 2014 Apr;454-455:371-9. doi: 10.1016/j.virol.2014.02.004. Epub 2014 Feb 20. Virology. 2014. PMID: 24559861 Free PMC article. Review. - Cytoplasmic dynein promotes HIV-1 uncoating.
Pawlica P, Berthoux L. Pawlica P, et al. Viruses. 2014 Nov 4;6(11):4195-211. doi: 10.3390/v6114195. Viruses. 2014. PMID: 25375884 Free PMC article.
References
- Anderson, K. B., H. A. Diep, and A. Zedeler. 2006. Murine leukemia virus transmembrane protein R-peptide is found in small virus core-like complexes in cells. J. Gen. Virol. 87:1583-1588. - PubMed
- Auewarakul, P., P. Wacharapornin, S. Srichatrapimuk, S. Chutipongtanate, and P. Puthavathana. 2005. Uncoating of HIV-1 requires cellular activation. Virology 337:93-101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI063987/AI/NIAID NIH HHS/United States
- R21 AI076094/AI/NIAID NIH HHS/United States
- AI063987/AI/NIAID NIH HHS/United States
- AI06354/AI/NIAID NIH HHS/United States
- AI076094/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources