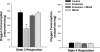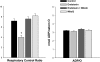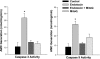MitoQ administration prevents endotoxin-induced cardiac dysfunction - PubMed (original) (raw)
MitoQ administration prevents endotoxin-induced cardiac dysfunction
G S Supinski et al. Am J Physiol Regul Integr Comp Physiol. 2009 Oct.
Abstract
Sepsis elicits severe alterations in cardiac function, impairing cardiac mitochondrial and pressure-generating capacity. Currently, there are no therapies to prevent sepsis-induced cardiac dysfunction. We tested the hypothesis that administration of a mitochondrially targeted antioxidant, 10-(6'-ubiquinonyl)-decyltriphenylphosphonium (MitoQ), would prevent endotoxin-induced reductions in cardiac mitochondrial and contractile function. Studies were performed on adult rodents (n = 52) given either saline, endotoxin (8 mg x kg(-1) x day(-1)), saline + MitoQ (500 microM), or both endotoxin and MitoQ. At 48 h animals were killed and hearts were removed for determination of either cardiac mitochondrial function (using polarography) or cardiac pressure generation (using the Langendorf technique). We found that endotoxin induced reductions in mitochondrial state 3 respiration rates, the respiratory control ratio, and ATP generation. Moreover, MitoQ administration prevented each of these endotoxin-induced abnormalities, P < 0.001. We also found that endotoxin produced reductions in cardiac pressure-generating capacity, reducing the systolic pressure-diastolic relationship. MitoQ also prevented endotoxin-induced reductions in cardiac pressure generation, P < 0.01. One potential link between mitochondrial and contractile dysfunction is caspase activation; we found that endotoxin increased cardiac levels of active caspases 9 and 3 (P < 0.001), while MitoQ prevented this increase (P < 0.01). These data demonstrate that MitoQ is a potent inhibitor of endotoxin-induced mitochondrial and cardiac abnormalities. We speculate that this agent may prove a novel therapy for sepsis-induced cardiac dysfunction.
Figures
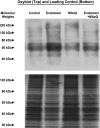
Fig. 1.
Cardiac OxyBlots. Top: OxyBlot determinations for cardiac samples from representative control, endotoxin, 10-(6′-ubiquinonyl)-decyltriphenylphosphonium (MitoQ), and endotoxin + MitoQ-treated animals. OxyBlot protein staining was more prominent for the endotoxin sample compared with the other groups. Bottom: a duplicate gel examining silver staining for all proteins contained in these samples; this serves as a loading control.
Fig. 2.
State 3 and state 4 respiration rates. State 3 respiration rate is a measure of maximum ADP-stimulated oxygen consumption, and state 4 respiration rate is a measure of basal oxygen consumption in the absence of ADP. Left: endotoxin administration elicited a large reduction in cardiac mitochondria state 3 rate (P < 0.001 for comparison of controls and to the endotoxin group), and administration of MitoQ prevented this endotoxin-induced reduction (P < 0.01 for comparison of the endotoxin group to the endotoxin + MitoQ group). Right: state 4 rates were similar for all experimental groups. *Statistically significant difference from the other groups.
Fig. 3.
Respiratory control and ADP-to-O ratios. The ADP-to-O ratio is an index of the coupling of oxygen consumption to oxidative phosphorylation. Endotoxin reduced the respiratory control ratio (RCR) (P < 0.001, for comparison of the control to the endotoxin group) but did not change the ADP-to-O ratio. Administration of MitoQ prevented this endotoxin-induced reduction in RCR (P < 0.01 for comparison of endotoxin to the endotoxin + MitoQ group). *Statistically significant difference from the other groups.
Fig. 4.
Cardiac caspase 3 (left) and caspase 9 (right) activity. Endotoxin induced a large increase in both cardiac caspase 3 and caspase 9 activities (P < 0.001 for comparison of control to endotoxin-treated groups). MitoQ administration blocked this endotoxin effect on caspase activities (P < 0.01 for comparison of caspase 3 between endotoxin and endotoxin + MitoQ groups; P < 0.01 for comparison of caspase 9 between these 2 groups). *Statistically significant difference from the other groups. AMC, amino-4-methylcoumarin.
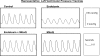
Fig. 5.
Representative left ventricular pressure tracings. The heart from an animal given endotoxin administration developed a much lower left ventricular pressure (right top tracing) compared with the pressure generated by a heart from a control animal (left top tracing). Developed left ventricular pressure for a heart from an animal given both endotoxin and MitoQ (left bottom tracing) was similar to the pressure in a control heart. Pressures for animals given MitoQ alone (right bottom tracing) were similar to control.
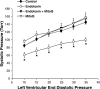
Fig. 6.
Systolic pressure-to-diastolic pressure relationship. Endotoxin-induced a significant reduction in developed left ventricular pressure for cardiac Langendorf preparations over the entire range of diastolic pressures assessed [P < 0.01 for comparison of control (•) to endotoxin (○)]. Hearts from animals given both endotoxin and MitoQ (▴) developed systolic pressures higher than those for the endotoxin group (P < 0.02). Systolic pressures for the hearts from animals given MitoQ alone (▵) were similar to controls. *Statistically significant difference from the other groups.
Similar articles
- Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction.
Supinski GS, Callahan LA. Supinski GS, et al. Am J Respir Crit Care Med. 2006 Jun 1;173(11):1240-7. doi: 10.1164/rccm.200410-1346OC. Epub 2006 Mar 2. Am J Respir Crit Care Med. 2006. PMID: 16514113 Free PMC article. - Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: Prevention by the targeted antioxidant MitoQ.
Vergeade A, Mulder P, Vendeville-Dehaudt C, Estour F, Fortin D, Ventura-Clapier R, Thuillez C, Monteil C. Vergeade A, et al. Free Radic Biol Med. 2010 Sep 1;49(5):748-56. doi: 10.1016/j.freeradbiomed.2010.05.024. Epub 2010 Jun 4. Free Radic Biol Med. 2010. PMID: 20566328 - Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice.
Goh KY, He L, Song J, Jinno M, Rogers AJ, Sethu P, Halade GV, Rajasekaran NS, Liu X, Prabhu SD, Darley-Usmar V, Wende AR, Zhou L. Goh KY, et al. Redox Biol. 2019 Feb;21:101100. doi: 10.1016/j.redox.2019.101100. Epub 2019 Jan 8. Redox Biol. 2019. PMID: 30641298 Free PMC article. - Mitoquinone mesylate (MitoQ) prevents sepsis-induced diaphragm dysfunction.
Supinski GS, Schroder EA, Wang L, Morris AJ, Callahan LAP. Supinski GS, et al. J Appl Physiol (1985). 2021 Aug 1;131(2):778-787. doi: 10.1152/japplphysiol.01053.2020. Epub 2021 Jul 1. J Appl Physiol (1985). 2021. PMID: 34197233 Free PMC article. - Animal and human studies with the mitochondria-targeted antioxidant MitoQ.
Smith RA, Murphy MP. Smith RA, et al. Ann N Y Acad Sci. 2010 Jul;1201:96-103. doi: 10.1111/j.1749-6632.2010.05627.x. Ann N Y Acad Sci. 2010. PMID: 20649545 Review.
Cited by
- Sepsis-induced Cardiac Mitochondrial Damage and Potential Therapeutic Interventions in the Elderly.
Zang QS, Wolf SE, Minei JP. Zang QS, et al. Aging Dis. 2014 Apr 1;5(2):137-49. doi: 10.14336/AD.2014.0500137. eCollection 2014 Apr. Aging Dis. 2014. PMID: 24729939 Free PMC article. Review. - Cellular metabolic and autophagic pathways: traffic control by redox signaling.
Dodson M, Darley-Usmar V, Zhang J. Dodson M, et al. Free Radic Biol Med. 2013 Oct;63:207-21. doi: 10.1016/j.freeradbiomed.2013.05.014. Epub 2013 May 20. Free Radic Biol Med. 2013. PMID: 23702245 Free PMC article. Review. - Innate Immunity as an Executor of the Programmed Death of Individual Organisms for the Benefit of the Entire Population.
Chernyak BV, Lyamzaev KG, Mulkidjanian AY. Chernyak BV, et al. Int J Mol Sci. 2021 Dec 15;22(24):13480. doi: 10.3390/ijms222413480. Int J Mol Sci. 2021. PMID: 34948277 Free PMC article. Review. - Sepsis-Induced Myocardial Dysfunction (SIMD): the Pathophysiological Mechanisms and Therapeutic Strategies Targeting Mitochondria.
Lin Y, Xu Y, Zhang Z. Lin Y, et al. Inflammation. 2020 Aug;43(4):1184-1200. doi: 10.1007/s10753-020-01233-w. Inflammation. 2020. PMID: 32333359 Review. - The mitochondria-targeted antioxidant mitoquinone protects against cold storage injury of renal tubular cells and rat kidneys.
Mitchell T, Rotaru D, Saba H, Smith RA, Murphy MP, MacMillan-Crow LA. Mitchell T, et al. J Pharmacol Exp Ther. 2011 Mar;336(3):682-92. doi: 10.1124/jpet.110.176743. Epub 2010 Dec 15. J Pharmacol Exp Ther. 2011. PMID: 21159749 Free PMC article.
References
- Adam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005 - PubMed
- Atamna H, Frey WH., II Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion 7: 297–310, 2007 - PubMed
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 - PubMed
- Callahan LA, Stofan D, Szweda L, Nethery D, Supinski GS. Free radicals alter maximal diaphragmatic oxygen consumption in endotoxin-induced sepsis. Free Radic Biol Med 30: 129–138, 2001 - PubMed
- Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med 172: 861–868, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-80429/HL/NHLBI NIH HHS/United States
- HL-80609/HL/NHLBI NIH HHS/United States
- HL-63698/HL/NHLBI NIH HHS/United States
- HL-81525/HL/NHLBI NIH HHS/United States
- HL-69821/HL/NHLBI NIH HHS/United States
- MC_U105663142/MRC_/Medical Research Council/United Kingdom
- R01 HL063698/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical