STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells - PubMed (original) (raw)
STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells
Maureen M Sherry et al. Stem Cells. 2009 Oct.
Abstract
Signal transducer and activator of transcription 3 (STAT3) regulates diverse cellular processes, including cell growth, differentiation, and apoptosis, and is frequently activated during tumorigenesis. Recently, putative glioblastoma stem cells (GBM-SCs) were isolated and characterized. These cells can self-renew indefinitely in culture, are highly tumorigenic, and retain the ability to differentiate in culture. We have found that treatment of GBM-SCs with two chemically distinct small molecule inhibitors of STAT3 DNA-binding inhibits cell proliferation and the formation of new neurospheres from single cells. Genetic knockdown of STAT3 using a short hairpin RNA also inhibits GBM-SC proliferation and neurosphere formation, confirming that these effects are specific to STAT3. Although STAT3 inhibition can induce apoptosis in serum-derived GBM cell lines, this effect was not observed in GBM-SCs grown in stem cell medium. Markers of neural stem cell multipotency also decrease upon STAT3 inhibition, suggesting that STAT3 is required for maintenance of the stem-like characteristics of these cells. Strikingly, even a transient inhibition of STAT3 leads to irreversible growth arrest and inhibition of neurosphere formation. These data suggest that STAT3 regulates the growth and self-renewal of GBM-SCs and is thus a potential target for cancer stem cell-directed therapy of glioblastoma multiforme.
Figures
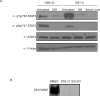
Figure 1. Glioblastoma stem cells derived from primary tumor samples form neurospheres in culture, express neural stem cell markers, and retain the ability to differentiate
A. Images of GS7-2 and GS6-22 neurospheres grown in serum-free media with EGF and FGF-2 (Untreated) or differentiated by plating on poly-L-ornithine and laminin in the presence of 2% serum without growth factors (Differentiated). B. Immunoblot of lysates of GS7-2 and GS-22 neurospheres in serum-free media or after 7 days under differentiation conditions with antibodies to CD133, nestin, and β-actin. C. RT-qPCR analysis of GFAP, βIII-tubulin, and olig2 mRNA in GS7-2 and GS6-22 neurospheres exposed to differentiation conditions for 7 days. Fold change values were calculated relative to untreated control and normalized by comparison to β-actin and plotted as log2. Bars represent mean of three separate experiments; bars SD.
Figure 2. STAT3 is expressed and activated in GBM stem cells
A. Immunoblot of STAT3, pSer727 STAT3, and pTyr705 STAT3 in GBM-SC untreated and differentiated for 7 days. The serum line derived from the GS7-2 parent tumor was also probed. B. Bandshift assay of GS7-2 neurosphere lysates treated with inhibitors of STAT3 DNA binding (STA-21 30μM, S3I-201 100 μM), incubated with radiolabeled high affinity SIE probe, and separated by PAGE.
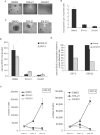
Figure 3. STAT3 inhibitors prevent neurosphere formation and proliferation of GBM stem cells
A. GS7-2 neurospheres mechanically dissociated into single cells, seeded into 96 well plates, and treated with the indicated STAT3 inhibitors or DMSO control. Representative images after 4 days in culture are shown (50X magnification). B. Quantitation of experiment described in A. The number of neurospheres in a single visual field (50X) per well was determined for 21 wells per treatment. Values represent mean; bars SD. C. STAT3 inhibitors cause dissociation of existing neurospheres. Intact GS6-22 spheres were treated with STAT3 inhibitors or DMSO control for 4 days. D. Inhibition of STAT3 prevents BrdU incorporation in GBM stem cells. GS6-22 and GS7-2 neurospheres were treated with STAT3 inhibitors for 24 hours and then pulsed for 16 hours with 10 μM BrdU. Cells were fixed and stained with FITC-conjugated anti-BrdU using the FITC-anti-BrdU Kit (BD Biosciences) and analyzed via flow cytometry. Bars represent SD of the mean of three separate experiments. E. Knockdown of STAT3 decreases BrdU incorporation in glioblastoma stem cells. GS6-22 and GS7-2 cells were infected with lentiviruses containing either shRNA to STAT3 or a non-targetting control shRNA. 3 days post-infection and puromycin selection, cells were pulsed with 10 μM BrdU for 3 days, fixed, stained, and analyzed as described above. Data is represented as the percent BrdU incorporation compared to control-infected cells. F. STAT3 inhibition prevents GBM-SC growth. GS6-22 and GS7-2 cells were dissociated into single cells and plated in equal numbers. 24 hours after plating, cells were counted and treated with DMSO, STA-21 and S3I-201. At day 3 and day 6 post-drug treatment cells were counted in triplicate, and plotted as SD of the mean.
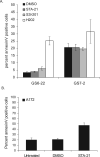
Figure 4. STAT3 inhibitor treatment causes increased apoptosis in serum-derived GBM cell lines, but not in GBM stem cells
A. GS7-2 and GS6-22 cells were treated with STAT3 inhibitor or DMSO control for 3 days, stained with annexinV-FITC, and analyzed via flow cytometry. H2O2 treatment (4.4 mM) was performed for 4 hours as a positive control. Cells were stained using the AnnexinV-FITC Apoptosis Detection Kit (BD Biosciences) according to the manufacturer's instructions. Values represent mean of three samples; bars SE. B. The A172 serum-derived glioma cell line was treated for 3 days with STA-21 or DMSO and analyzed as described above. Values represent mean of three experiments; bars SE.
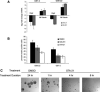
Figure 5. STAT3 inhibition depletes markers of stem-ness in GBM stem cells
A. Olig2 and βIII-tubulin transcript levels in GBM-SC treated with STAT3 inhibitors or DMSO control for 7 days. mRNA was quantified by RT-qPCR. Data is shown as fold change relative to DMSO-treated cells and normalized for β-actin. Values represent mean of three experiments and bars SD. B. Nestin expression in GS7-2 and GS6-22 GBM-SC treated for 7 days with STAT3 inhibitors or DMSO control. Expression was quantified by immunostaining of fixed cells with mouse α-nestin antibody followed by flow cytometry. C. Treatment with STAT3 inhibitor STA-21 irreversibly inhibits neurosphere formation. GS6-22 cells were treated with inhibitor for the indicated periods of time, washed thoroughly, and replated in fresh media. Representative images were taken one week after drug removal.
Similar articles
- Effect of the STAT3 inhibitor STX-0119 on the proliferation of cancer stem-like cells derived from recurrent glioblastoma.
Ashizawa T, Miyata H, Iizuka A, Komiyama M, Oshita C, Kume A, Nogami M, Yagoto M, Ito I, Oishi T, Watanabe R, Mitsuya K, Matsuno K, Furuya T, Okawara T, Otsuka M, Ogo N, Asai A, Nakasu Y, Yamaguchi K, Akiyama Y. Ashizawa T, et al. Int J Oncol. 2013 Jul;43(1):219-27. doi: 10.3892/ijo.2013.1916. Epub 2013 Apr 23. Int J Oncol. 2013. PMID: 23612755 - The effects of type I interferon on glioblastoma cancer stem cells.
Du Z, Cai C, Sims M, Boop FA, Davidoff AM, Pfeffer LM. Du Z, et al. Biochem Biophys Res Commun. 2017 Sep 16;491(2):343-348. doi: 10.1016/j.bbrc.2017.07.098. Epub 2017 Jul 18. Biochem Biophys Res Commun. 2017. PMID: 28728846 - Stattic and metformin inhibit brain tumor initiating cells by reducing STAT3-phosphorylation.
Leidgens V, Proske J, Rauer L, Moeckel S, Renner K, Bogdahn U, Riemenschneider MJ, Proescholdt M, Vollmann-Zwerenz A, Hau P, Seliger C. Leidgens V, et al. Oncotarget. 2017 Jan 31;8(5):8250-8263. doi: 10.18632/oncotarget.14159. Oncotarget. 2017. PMID: 28030813 Free PMC article. - The role of STAT3 in glioblastoma progression through dual influences on tumor cells and the immune microenvironment.
Chang N, Ahn SH, Kong DS, Lee HW, Nam DH. Chang N, et al. Mol Cell Endocrinol. 2017 Aug 15;451:53-65. doi: 10.1016/j.mce.2017.01.004. Epub 2017 Jan 12. Mol Cell Endocrinol. 2017. PMID: 28089821 Review. - STAT3 as a Therapeutic Target for Glioblastoma.
Liu Y, Li C, Lin J. Liu Y, et al. Anticancer Agents Med Chem. 2010 Sep;10(7):512-9. doi: 10.2174/187152010793498636. Anticancer Agents Med Chem. 2010. PMID: 20879983 Review.
Cited by
- CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine.
Gu Y, Chen T, Meng Z, Gan Y, Xu X, Lou G, Li H, Gan X, Zhou H, Tang J, Xu G, Huang L, Zhang X, Fang Y, Wang K, Zheng S, Huang W, Xu R. Gu Y, et al. Blood. 2012 Dec 6;120(24):4829-39. doi: 10.1182/blood-2012-06-434894. Epub 2012 Oct 16. Blood. 2012. PMID: 23074277 Free PMC article. - HCMV activates the IL-6-JAK-STAT3 axis in HepG2 cells and primary human hepatocytes.
Lepiller Q, Abbas W, Kumar A, Tripathy MK, Herbein G. Lepiller Q, et al. PLoS One. 2013;8(3):e59591. doi: 10.1371/journal.pone.0059591. Epub 2013 Mar 26. PLoS One. 2013. PMID: 23555719 Free PMC article. - miRNA-194-3p represses NF-κB in gliomas to attenuate iPSC genes and proneural to mesenchymal transition.
Jacob JR, Singh R, Okamoto M, Chakravarti A, Palanichamy K. Jacob JR, et al. iScience. 2023 Dec 7;27(1):108650. doi: 10.1016/j.isci.2023.108650. eCollection 2024 Jan 19. iScience. 2023. PMID: 38226170 Free PMC article. - Delineating the cytogenomic and epigenomic landscapes of glioma stem cell lines.
Baronchelli S, Bentivegna A, Redaelli S, Riva G, Butta V, Paoletta L, Isimbaldi G, Miozzo M, Tabano S, Daga A, Marubbi D, Cattaneo M, Biunno I, Dalprà L. Baronchelli S, et al. PLoS One. 2013;8(2):e57462. doi: 10.1371/journal.pone.0057462. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23468990 Free PMC article. - Chemoresistance, cancer stem cells, and miRNA influences: the case for neuroblastoma.
Buhagiar A, Ayers D. Buhagiar A, et al. Anal Cell Pathol (Amst). 2015;2015:150634. doi: 10.1155/2015/150634. Epub 2015 Jul 14. Anal Cell Pathol (Amst). 2015. PMID: 26258008 Free PMC article. Review.
References
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. CANCER RES. 2003;63:5821–5828. - PubMed
- Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. ONCOGENE. 2004;23:9392–9400. - PubMed
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. CANCER RES. 2004;64:7011–7021. - PubMed
- Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. GLIA. 2002;39:193–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047243/NS/NINDS NIH HHS/United States
- T32 DK007542/DK/NIDDK NIH HHS/United States
- T32 HD049341/HD/NICHD NIH HHS/United States
- T32 DK07542/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous