X-ray emission spectroscopy to study ligand valence orbitals in Mn coordination complexes - PubMed (original) (raw)
X-ray emission spectroscopy to study ligand valence orbitals in Mn coordination complexes
Grigory Smolentsev et al. J Am Chem Soc. 2009.
Abstract
We discuss a spectroscopic method to determine the character of chemical bonding and for the identification of metal ligands in coordination and bioinorganic chemistry. It is based on the analysis of satellite lines in X-ray emission spectra that arise from transitions between valence orbitals and the metal ion 1s level (valence-to-core XES). The spectra, in connection with calculations based on density functional theory (DFT), provide information that is complementary to other spectroscopic techniques, in particular X-ray absorption (XANES and EXAFS). The spectral shape is sensitive to protonation of ligands and allows ligands, which differ only slightly in atomic number (e.g., C, N, O...), to be distinguished. A theoretical discussion of the main spectral features is presented in terms of molecular orbitals for a series of Mn model systems: [Mn(H(2)O)(6)](2+), [Mn(H(2)O)(5)OH](+), and [Mn(H(2)O)(5)NH(3)](2+). An application of the method, with comparison between theory and experiment, is presented for the solvated Mn(2+) ion in water and three Mn coordination complexes, namely [LMn(acac)N(3)]BPh(4), [LMn(B(2)O(3)Ph(2))(ClO(4))], and [LMn(acac)N]BPh(4), where L represents 1,4,7-trimethyl-1,4,7-triazacyclononane, acac stands for the 2,4-pentanedionate anion, and B(2)O(3)Ph(2) represents the 1,3-diphenyl-1,3-dibora-2-oxapropane-1,3-diolato dianion.
Figures
Figure 1
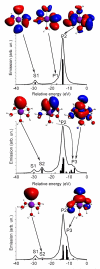
Theoretical valence-to-core XES (left panel) and Mn K-edge XANES (right panel) of [Mn(H2O)6]2+ (black solid line), [Mn(H2O)5OH]+ (red dash-dotted line) and [Mn(H2O)5NH3]2+ (blue dashed line). Spectra are shown with optimized local structures (top) and with fixed metal-ligand distances (bottom).
Figure 2
Theoretical valence-to-core XES, with some important molecular orbitals contributing to the spectra of [Mn(H2O)6]2+ (top), [Mn(H2O)5OH]+ (middle) and [Mn(H2O)5NH3]2+ (bottom). Sticks show contributions of individual molecular orbitals. Isosurfaces are colored red (blue) for positive (negative) values of the wavefunction. The geometry of the molecule is shown with O (red), H (grey), N (blue) and Mn (magenta).
Fig. 3
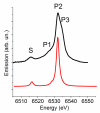
Experimental (top) valence-to-core XES of solvated Mn2+ ion in water and theoretical spectrum of [Mn(H2O)6]2+ (bottom).
Figure 4
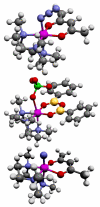
Schematic representation of [LMn(acac)N3]BPh4 (top) and [LMn(B2O3Ph2)(ClO4)] (middle) and [LMn(acac)N] BPh4 (bottom) with C (black), O (red), N (blue), B (yellow), Cl (green), H (grey) and Mn (magenta) atoms. Distant counter ion BPh4 is not shown for the top and bottom structures.
Figure 5
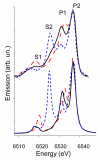
Experimental (top) and theoretical (bottom) valence-to-core XES of [LMn(acac)N3]BPh4 (black solid lines), [LMn(B2O3Ph2)(ClO4)] (red dashed lines) and [LMn(acac)N]BPh4 (blue short-dashed lines).
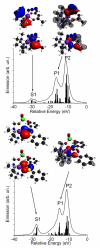
Figure 6
Theoretical valence-to-core XES and some important molecular orbitals contributing to the XES spectra of [LMn(acac)N3]BPh4 (top) and [LMn(B2O3Ph2)(ClO4)] (bottom). Sticks show contributions of individual molecular orbitals. Isosurfaces corresponding to the positive (red) and negative (blue) values of the wave function are plotted. Parts of the MOs are shown as a mesh for clarity. The geometry of the molecules is shown with O (red), N (blue), C (black), B (yellow), Cl (green), H (grey) and Mn (magenta) atoms.
Similar articles
- Insights into the geometric and electronic structure of transition metal centers from valence-to-core X-ray emission spectroscopy.
Pollock CJ, DeBeer S. Pollock CJ, et al. Acc Chem Res. 2015 Nov 17;48(11):2967-75. doi: 10.1021/acs.accounts.5b00309. Epub 2015 Sep 24. Acc Chem Res. 2015. PMID: 26401686 - Experimental and computational X-ray emission spectroscopy as a direct probe of protonation states in oxo-bridged Mn(IV) dimers relevant to redox-active metalloproteins.
Lassalle-Kaiser B, Boron TT 3rd, Krewald V, Kern J, Beckwith MA, Delgado-Jaime MU, Schroeder H, Alonso-Mori R, Nordlund D, Weng TC, Sokaras D, Neese F, Bergmann U, Yachandra VK, DeBeer S, Pecoraro VL, Yano J. Lassalle-Kaiser B, et al. Inorg Chem. 2013 Nov 18;52(22):12915-22. doi: 10.1021/ic400821g. Epub 2013 Oct 25. Inorg Chem. 2013. PMID: 24161081 Free PMC article. - Ligand identification in titanium complexes using X-ray valence-to-core emission spectroscopy.
Swarbrick JC, Kvashnin Y, Schulte K, Seenivasan K, Lamberti C, Glatzel P. Swarbrick JC, et al. Inorg Chem. 2010 Sep 20;49(18):8323-32. doi: 10.1021/ic100755t. Inorg Chem. 2010. PMID: 20831281 - High-resolution X-ray spectroscopy of rare events: a different look at local structure and chemistry.
Bergmann U, Glatzel P, Robblee JH, Messinger J, Fernandez C, Cinco R, Visser H, McFarlane K, Bellacchio E, Pizarro S, Sauer K, Yachandra VK, Klein MP, Cox BL, Nealson KH, Cramer SP. Bergmann U, et al. J Synchrotron Radiat. 2001 Mar 1;8(Pt 2):199-203. doi: 10.1107/s0909049500016484. J Synchrotron Radiat. 2001. PMID: 11512725 Free PMC article. Review. - X-ray spectroscopy of the Mn4Ca cluster in the water-oxidation complex of Photosystem II.
Sauer K, Yano J, Yachandra VK. Sauer K, et al. Photosynth Res. 2005;85(1):73-86. doi: 10.1007/s11120-005-0638-9. Photosynth Res. 2005. PMID: 15977060 Free PMC article. Review.
Cited by
- The Fe-V Cofactor of Vanadium Nitrogenase Contains an Interstitial Carbon Atom.
Rees JA, Bjornsson R, Schlesier J, Sippel D, Einsle O, DeBeer S. Rees JA, et al. Angew Chem Int Ed Engl. 2015 Nov 2;54(45):13249-52. doi: 10.1002/anie.201505930. Epub 2015 Sep 17. Angew Chem Int Ed Engl. 2015. PMID: 26376620 Free PMC article. - Feasibility of Valence-to-Core X-ray Emission Spectroscopy for Tracking Transient Species.
March AM, Assefa TA, Bressler C, Doumy G, Galler A, Gawelda W, Kanter EP, Németh Z, Pápai M, Southworth SH, Young L, Vankó G. March AM, et al. J Phys Chem C Nanomater Interfaces. 2015 Jul 2;119(26):14571-14578. doi: 10.1021/jp511838q. Epub 2015 Feb 9. J Phys Chem C Nanomater Interfaces. 2015. PMID: 26568779 Free PMC article. - Direct detection of oxygen ligation to the Mn(4)Ca cluster of photosystem II by X-ray emission spectroscopy.
Pushkar Y, Long X, Glatzel P, Brudvig GW, Dismukes GC, Collins TJ, Yachandra VK, Yano J, Bergmann U. Pushkar Y, et al. Angew Chem Int Ed Engl. 2010;49(4):800-3. doi: 10.1002/anie.200905366. Angew Chem Int Ed Engl. 2010. PMID: 20017172 Free PMC article. No abstract available. - Element substitution by living organisms: the case of manganese in mollusc shell aragonite.
Soldati AL, Jacob DE, Glatzel P, Swarbrick JC, Geck J. Soldati AL, et al. Sci Rep. 2016 Mar 9;6:22514. doi: 10.1038/srep22514. Sci Rep. 2016. PMID: 26957325 Free PMC article. - Probing a Silent Metal: A Combined X-ray Absorption and Emission Spectroscopic Study of Biologically Relevant Zinc Complexes.
McCubbin Stepanic O, Ward J, Penner-Hahn JE, Deb A, Bergmann U, DeBeer S. McCubbin Stepanic O, et al. Inorg Chem. 2020 Sep 21;59(18):13551-13560. doi: 10.1021/acs.inorgchem.0c01931. Epub 2020 Sep 6. Inorg Chem. 2020. PMID: 32893611 Free PMC article.
References
- Bergquist C, Fillebeen T, Morlok M, Parkin G. J. Am. Chem. Soc. 2003;125:6189–6199. - PubMed
- Stone EM, Costello AL, Tierney DL, Fasr W. Biochemistry. 2006;45:5618–5630. - PubMed
- Kaminskaia NV, Spingler B, Lippard SJ. J. Am.Chem. Soc. 2000;122:6411–6422.
- Zampella G, Fantucci P, Pecoraro VL, Geoia LD. J. Am. Chem. Soc. 2005;127:953–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM055302/GM/NIGMS NIH HHS/United States
- R01 GM055302-03/GM/NIGMS NIH HHS/United States
- R56 GM055302/GM/NIGMS NIH HHS/United States
- GM 55302/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous