The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming - PubMed (original) (raw)
Review
The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming
Amar M Singh et al. Cell Stem Cell. 2009.
Abstract
Pluripotent stem cells have long-term proliferative capacity and an unusual mode of cell-cycle regulation and can divide independently of extrinsic mitogenic signals. The last few years has seen evidence emerge that links cell-cycle regulation to the maintenance and establishment of pluripotency. Myc transcription factors appear to be central to this regulation. This review addresses these links and discusses how cell-cycle controls and Myc impact on the maintenance and establishment of pluripotency.
Figures
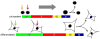
Figure 1. Cell cycle structure of human and murine pluripotent cells and fibroblasts
This figure represents typical cell cycle profiles from a range of pluripotent cell types (mESC, hESC, miPSC, hiPSC and mEpiSC) and primary fibroblasts. Pluripotent cells spend a high proportion of time in S-phase cells and a low proportion of time in G1 (Savatier et al., 1996; Stead et al., 2002; Fluckiger et al., 2006; Ohtsuka and Dalton, 2008; Dalton, 2009; Neganova et al., 2009; Zhang et al., 2009). MEFs and human IMR90 fibroblasts show a lower % of cells in S-phase and an increased proportion in G1, relative to pluripotent cells. hPRCs display a cell cycle structure intermediate between differentiated fibroblasts and pluripotent cells. The positions of G1 cells (2n DNA content), S-phase cells and G2/M cells (4n DNA content) are indicated. The Y-axis represents the relative number of cells and the X-axis represents the DNA content of cells, a read-out for cell cycle position.
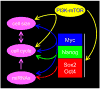
Figure 2. The relationships between pluripotency, cell cycle and cell size control
The figure represents a summary of the pathways discussed throughout this review, now thought to be important for cell cycle and cell size control in pluripotent cells. The core transcriptional network inherent to pluripotent stem cells impacts on this network at several points. Evidence links Myc to all major points in the regulatory network. For example, Myc regulates the cell cycle directly through regulation of Cdk activity and indirectly through miRNAs. We speculate that accelerated progress through G1 can account for the small size of pluripoent cells. Interestingly, cells with inactive Rb-family members progress through G1 rapidly and have reduced cell volume.
Figure 3. How Myc and cell cycle regulatory controls intersect with different stages of reprogramming
Cells subject to reprogramming cues undergo a three step process as they transition towards iPSCs. The initial step involves Myc which causes global changes in genes expression, including repression of differentiation genes, without induction of pluripotency genes (see Sridharan et al., 2009; Zhao and Daley et al., 2008; Mahereli et al., 2008; Judson et al., 2009; Silva et al., 2008; Mikkelesen et al., 2008). Other reprogramming factors, possibly in combination with Myc (Huangfu et al., 2008), then drive the formation of partially reprogrammed cells (PRCs) and then fully reprogrammed iPSCs. Roles for Myc have also been defined in the maintenance of iPSCs and other pluripotent cell types, some of which are directly related to cell cycle control (Cartwright et al., 2005; Wang et al., 2008; Judson et al., 2009; Hanna et al., 2009). Possible points at which Myc could be involved, but for which there are no solid data, are indicated by broken arrows. For example, it is likely that for reprogramming cells need to be constantly kept cycling by Myc.
Similar articles
- Myc represses primitive endoderm differentiation in pluripotent stem cells.
Smith KN, Singh AM, Dalton S. Smith KN, et al. Cell Stem Cell. 2010 Sep 3;7(3):343-54. doi: 10.1016/j.stem.2010.06.023. Cell Stem Cell. 2010. PMID: 20804970 Free PMC article. - Roles for MYC in the establishment and maintenance of pluripotency.
Chappell J, Dalton S. Chappell J, et al. Cold Spring Harb Perspect Med. 2013 Dec 1;3(12):a014381. doi: 10.1101/cshperspect.a014381. Cold Spring Harb Perspect Med. 2013. PMID: 24296349 Free PMC article. Review. - Regeneration and reprogramming compared.
Christen B, Robles V, Raya M, Paramonov I, Izpisúa Belmonte JC. Christen B, et al. BMC Biol. 2010 Jan 20;8:5. doi: 10.1186/1741-7007-8-5. BMC Biol. 2010. PMID: 20089153 Free PMC article. - Quick, Coordinated and Authentic Reprogramming of Ribosome Biogenesis during iPSC Reprogramming.
Hu K. Hu K. Cells. 2020 Nov 15;9(11):2484. doi: 10.3390/cells9112484. Cells. 2020. PMID: 33203179 Free PMC article. - Why myc? An unexpected ingredient in the stem cell cocktail.
Knoepfler PS. Knoepfler PS. Cell Stem Cell. 2008 Jan 10;2(1):18-21. doi: 10.1016/j.stem.2007.12.004. Cell Stem Cell. 2008. PMID: 18371417 Review.
Cited by
- The shifting shape and functional specializations of the cell cycle during lineage development.
Hwang Y, Hidalgo D, Socolovsky M. Hwang Y, et al. WIREs Mech Dis. 2021 Mar;13(2):e1504. doi: 10.1002/wsbm.1504. Epub 2020 Sep 11. WIREs Mech Dis. 2021. PMID: 32916032 Free PMC article. Review. - CCND1-CDK4-mediated cell cycle progression provides a competitive advantage for human hematopoietic stem cells in vivo.
Mende N, Kuchen EE, Lesche M, Grinenko T, Kokkaliaris KD, Hanenberg H, Lindemann D, Dahl A, Platz A, Höfer T, Calegari F, Waskow C. Mende N, et al. J Exp Med. 2015 Jul 27;212(8):1171-83. doi: 10.1084/jem.20150308. Epub 2015 Jul 6. J Exp Med. 2015. PMID: 26150472 Free PMC article. - Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal.
Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith A. Martello G, et al. Cell Stem Cell. 2012 Oct 5;11(4):491-504. doi: 10.1016/j.stem.2012.06.008. Cell Stem Cell. 2012. PMID: 23040478 Free PMC article. - Reprogramming mechanisms influence the maturation of hematopoietic progenitors from human pluripotent stem cells.
Heo HR, Song H, Kim HR, Lee JE, Chung YG, Kim WJ, Yang SR, Kim KS, Chun T, Lee DR, Hong SH. Heo HR, et al. Cell Death Dis. 2018 Oct 24;9(11):1090. doi: 10.1038/s41419-018-1124-6. Cell Death Dis. 2018. PMID: 30356076 Free PMC article. - Destroy to create: E3 ubiquitin ligases in neurogenesis.
Stegmüller J, Bonni A. Stegmüller J, et al. F1000 Biol Rep. 2010 May 24;2:38. doi: 10.3410/B2-38. F1000 Biol Rep. 2010. PMID: 20948796 Free PMC article.
References
- Alvarez B, Garrido E, Garcia-Sanz JA, Carrera AC. Phosphoinositide 3-kinase activation regulates cell division time by coordinated control of cell mass and cell cycle progression rate. J Biol Chem. 2003;278:26466–26473. - PubMed
- Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–268. - PubMed
- Baserga R. Is cell size important? Cell Cycle. 2007;6:814–816. - PubMed
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. - PubMed
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD049647/HD/NICHD NIH HHS/United States
- P01 GM085354-01/GM/NIGMS NIH HHS/United States
- P01 GM085354-02S10001/GM/NIGMS NIH HHS/United States
- GM75334/GM/NIGMS NIH HHS/United States
- P01 GM085354/GM/NIGMS NIH HHS/United States
- P01 GM085354-020001/GM/NIGMS NIH HHS/United States
- P01 GM085354-02S1/GM/NIGMS NIH HHS/United States
- P01 GM085354-010001/GM/NIGMS NIH HHS/United States
- P01 GM085354-03/GM/NIGMS NIH HHS/United States
- R01 HD049647/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources