Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer - PubMed (original) (raw)
Comparative Study
. 2009 Sep 1;183(5):3425-32.
doi: 10.4049/jimmunol.0900305. Epub 2009 Aug 10.
Affiliations
- PMID: 19667085
- PMCID: PMC2860795
- DOI: 10.4049/jimmunol.0900305
Comparative Study
Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer
Mohd W Nasser et al. J Immunol. 2009.
Abstract
CXCL8 (also known as IL-8) activates CXCR1 and CXCR2 to mediate neutrophil recruitment and trigger cytotoxic effect at sites of infection. Under physiological conditions, CXCL8 could exist as monomers, dimers, or a mixture of monomers and dimers. Therefore, both forms of CXCL8 could interact with CXCR1 and CXCR2 with different affinities and potencies to mediate different cellular responses. In the present study, we have used a "trapped" nonassociating monomer (L25NMe) and a nondissociating dimer (R26C) to investigate their activities for human neutrophils that express both receptors and for RBL-2H3 cells stably expressing either CXCR1(RBL-CXCR1) or CXCR2 (RBL-CXCR2). The monomer was more active than the dimer for activities such as intracellular Ca(2+) mobilization, phosphoinositide hydrolysis, chemotaxis. and exocytosis. Receptor regulation, however, is distinct for each receptor. The rate of monomer-mediated regulation of CXCR1 is greater for activities such as phosphorylation, desensitization, beta-arrestin translocation, and internalization. In contrast, for CXCR2, both monomeric and dimeric CXCL8 mediate these activities to a similar extent. Interestingly, receptor-mediated signal-regulated kinase (ERK) phosphorylation in response to all three CXCL8 variants was more sustained for CXCR2 relative to CXCR1. Taken together, the results indicate that the CXCL8 monomer and dimer differentially activate and regulate CXCR1 and CXCR2 receptors. These distinct properties of the ligand and the receptors play a critical role in orchestrating neutrophil recruitment and eliciting cytotoxic activity during an inflammatory response.
Figures
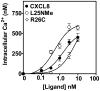
Figure 1. Monomer, dimer or native CXCL8 induced intracellular Ca2+ mobilization in human neutrophils
Human neutrophils (5 × 106) were loaded with indo-1AM and stimulated with different concentration of monomer, dimer, and WT CXCL8. The results are representative of one of three experiments performed in triplicate.
Figure 2. Expression and characterization of WT CXCR1 and CXCR2
A) A representative histogram of FACS analysis showing surface expression of CXCR1 and CXCR2 in RBL-2H3 cells after staining with CXCR1 (dotted line) or CXCR2 (broad line) specific antibodies. B) For intracellular Ca2+ mobilization, RBL cells (5 × 106) expressing human CXCR1 (RBL-CXCR1) or CXCR2 (RBL-CXCR2) were loaded with Indo-1AM and stimulated with monomer, dimer, or WT CXCL8. The representative tracings are one of the three experiments.
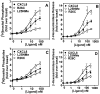
Figure 3. Phosphoinositide hydrolysis (PI), secretion of β-hexosaminidase release and Chemotaxis
For the generation of inositol phosphates (A and C), cells were cultured overnight in the presence of [3H]inositol (1 μC/ml). Cells were preincubated for 10 min at 37°C with HEPES-buffered saline containing 10 mM LiCl in a total volume of 200 μl and stimulated with different concentrations of monomer, dimer, or WT CXCL8 for 10 min. The supernatant was used to determine the release of inositol phosphates. Data are represented as the fold stimulation over basal. For secretion of β-hexosaminidase (B and D), cells were seeded as indicated above and stimulated with different concentrations of CXCL8 variants for 10 min. The supernatant (15 μl) was removed and β-hexosaminidase release was measured. Data are represented as fold stimulation over basal. Both experiments were repeated three times with similar results. Chemotactic response to CXCL8 variants using RBL-CXCR1 and RBL-CXCR2 (E and F) or HMECs (G and H) were measured as described in Materials and Methods. The results are representative of one of four experiments performed in triplicate. *, P< 0.05 and ** P< 0.01.
Figure 3. Phosphoinositide hydrolysis (PI), secretion of β-hexosaminidase release and Chemotaxis
For the generation of inositol phosphates (A and C), cells were cultured overnight in the presence of [3H]inositol (1 μC/ml). Cells were preincubated for 10 min at 37°C with HEPES-buffered saline containing 10 mM LiCl in a total volume of 200 μl and stimulated with different concentrations of monomer, dimer, or WT CXCL8 for 10 min. The supernatant was used to determine the release of inositol phosphates. Data are represented as the fold stimulation over basal. For secretion of β-hexosaminidase (B and D), cells were seeded as indicated above and stimulated with different concentrations of CXCL8 variants for 10 min. The supernatant (15 μl) was removed and β-hexosaminidase release was measured. Data are represented as fold stimulation over basal. Both experiments were repeated three times with similar results. Chemotactic response to CXCL8 variants using RBL-CXCR1 and RBL-CXCR2 (E and F) or HMECs (G and H) were measured as described in Materials and Methods. The results are representative of one of four experiments performed in triplicate. *, P< 0.05 and ** P< 0.01.
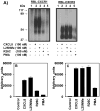
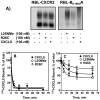
Figure 4. Phosphorylation of CXCR1 and CXCR2
A) 32P-labeled RBL-2H3 cells expressing CXCR1 or CXCR2were incubated for 5 min with or without stimulants as shown. Cells were lysed, immunoprecipitated with specific antibodies against CXCR1 or CXCR2 and then analyzed by SDS-PAGE and autoradiography. The results are from a representative experiment that was repeated three times. B and C, The amount of radioactivity per lane was determined by counting excised phosphorylated bands.
Figure 5. Internalization CXCR1 and CXCR2
RBL-CXCR1 (A) or RBL-CXCR2 (B) cells were treated with 100 nM of monomer, dimer, or WT CXCL8 for different times, washed and assayed for 125I-WT CXCL8 (0.1nM) binding. The value is presented as a percentage of totals, which is defined as the total amount of 125I-WT CXCL8 bound to control (untreated) cells. The experiment was repeated five times with similar results.
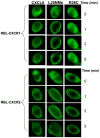
Figure 6. CXCR1 and CXCR2-mediated βarrestin 1 translocation to the cell membrane
RBL-CXCR1 and RBL-CXCR2 were transfected with β-arrestin1-GFP. The fluorescence images of GFP were captured upon addition of 100 nM of monomer, dimer, or WT CXCL8 to live cells as described in the method section.
Figure 7. CXCR1 and CXCR2-mediated ERK phosphorylation
RBL-2H3 cells stably expressing CXCR1 or CXCR2 were stimulated with monomer (D), dimmer(G), or WT CXCL8(A) (100 nM) for 0–5 min. ERK1/2 phosphorylation and total ERK were determined by Western blotting using anti-phospho-ERK1/2 (pERK1/2) and anti-total ERK1/2 (ERK1/2) antibodies, respectively. The experiments were repeated three times with similar results(B, C, E, F, H and I). Band density from radiogram was calculated by Image-Pro Plus software (Media Cybernetics) and is represented as relative phospho-ERK1/2 expression (arbitrary units). Data shown are averages of three experiments.
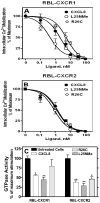
Figure 8. Desensitization of CXCR1 and CXCR2-mediated intracellular calcium mobilization and GTPase activity
RBL-2H3 cells (5×106 cells) expressing (A) CXCR1 or (B) CXCR2 were loaded with Indo-1 AM and exposed to a first dose of either monomer, dimer, or WT CXCL8 (0–100 nM). Cells were rechallenged 3 min later with a second dose of CXCL8 (10 nM) and intracellular Ca2+ mobilization was measured. Data are represented as percentage of maximum which represents intracellular Ca2+ mobilization-induced by CXCL8 (10 nM) in the absence of pretreatment (320±49 and 227±28 for RBL-CXCR1 and RBL-CXCR2, respectively). C) For GTPase activity, the cells were treated with or without ligand (100 nM) for 5 min. Membranes were prepared and assayed for WT CXCL8 (100 nM) stimulated GTP hydrolysis. The data are presented as percentage of maximum which is the net maximal stimulation obtained with untreated cells. Data shown are representative of three experiments performed in triplicate. *, P< 0.05 and ** P< 0.01.
Figure 9. Phosphorylation and internalization of BD199VA
32P-labeled RBL-2H3 cells (5 × 106 cells/60 mm plate) expressing CXCR2 (A, left panel) or BD199VA (A, right panel)were incubated for 5 min with or without stimulants as shown. Cells were lysed, immunoprecipitated with specific antibody against CXCR2 and then analyzed by SDS-PAGE and autoradiography. The results are from a representative experiment that was repeated twice. For internalization (panels B and C), cells were treated with 100 nM of ligands for different times, washed and assayed for 125I-WT CXCL8 (0.1nM) binding. The values are presented as a percentage of totals, which is defined as the total amount of 125I-WT CXCL8 bound to control (untreated) cells. The experiment was repeated three times with similar results.
Similar articles
- The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions.
Raghuwanshi SK, Su Y, Singh V, Haynes K, Richmond A, Richardson RM. Raghuwanshi SK, et al. J Immunol. 2012 Sep 15;189(6):2824-32. doi: 10.4049/jimmunol.1201114. Epub 2012 Aug 6. J Immunol. 2012. PMID: 22869904 Free PMC article. - Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation.
Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Richardson RM, et al. J Immunol. 2003 Mar 15;170(6):2904-11. doi: 10.4049/jimmunol.170.6.2904. J Immunol. 2003. PMID: 12626541 - Cross-desensitization among CXCR1, CXCR2, and CCR5: role of protein kinase C-epsilon.
Nasser MW, Marjoram RJ, Brown SL, Richardson RM. Nasser MW, et al. J Immunol. 2005 Jun 1;174(11):6927-33. doi: 10.4049/jimmunol.174.11.6927. J Immunol. 2005. PMID: 15905535 - Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation.
Planagumà A, Domènech T, Pont M, Calama E, García-González V, López R, Aulí M, López M, Fonquerna S, Ramos I, de Alba J, Nueda A, Prats N, Segarra V, Miralpeix M, Lehner MD. Planagumà A, et al. Pulm Pharmacol Ther. 2015 Oct;34:37-45. doi: 10.1016/j.pupt.2015.08.002. Epub 2015 Aug 10. Pulm Pharmacol Ther. 2015. PMID: 26271598 Review. - The CXCL8-CXCR1/2 pathways in cancer.
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K. Liu Q, et al. Cytokine Growth Factor Rev. 2016 Oct;31:61-71. doi: 10.1016/j.cytogfr.2016.08.002. Epub 2016 Aug 25. Cytokine Growth Factor Rev. 2016. PMID: 27578214 Free PMC article. Review.
Cited by
- Selective loss of chemokine receptor expression on leukocytes after cell isolation.
Nieto JC, Cantó E, Zamora C, Ortiz MA, Juárez C, Vidal S. Nieto JC, et al. PLoS One. 2012;7(3):e31297. doi: 10.1371/journal.pone.0031297. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403612 Free PMC article. - Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies.
Kufareva I, Salanga CL, Handel TM. Kufareva I, et al. Immunol Cell Biol. 2015 Apr;93(4):372-83. doi: 10.1038/icb.2015.15. Epub 2015 Feb 24. Immunol Cell Biol. 2015. PMID: 25708536 Free PMC article. Review. - MicroRNA hsa-miR-623 directly suppresses MMP1 and attenuates IL-8-induced metastasis in pancreatic cancer.
Chen Y, Peng S, Cen H, Lin Y, Huang C, Chen Y, Shan H, Su Y, Zeng L. Chen Y, et al. Int J Oncol. 2019 Jul;55(1):142-156. doi: 10.3892/ijo.2019.4803. Epub 2019 May 17. Int J Oncol. 2019. PMID: 31115512 Free PMC article. - Primary 1,25-dihydroxyvitamin D3 response of the interleukin 8 gene cluster in human monocyte- and macrophage-like cells.
Ryynänen J, Carlberg C. Ryynänen J, et al. PLoS One. 2013 Oct 21;8(10):e78170. doi: 10.1371/journal.pone.0078170. eCollection 2013. PLoS One. 2013. PMID: 24250750 Free PMC article. - G Protein-coupled receptor kinase-6 interacts with activator of G protein signaling-3 to regulate CXCR2-mediated cellular functions.
Singh V, Raghuwanshi SK, Smith N, Rivers EJ, Richardson RM. Singh V, et al. J Immunol. 2014 Mar 1;192(5):2186-94. doi: 10.4049/jimmunol.1301875. Epub 2014 Feb 7. J Immunol. 2014. PMID: 24510965 Free PMC article.
References
- Baggiolini M. Reflections on chemokines. Immunol Rev. 2000;177:5–7. - PubMed
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. - PubMed
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
- Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830–23836. - PubMed
- Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA156735/CA/NCI NIH HHS/United States
- 056-CA92077/CA/NCI NIH HHS/United States
- U56 CA092077/CA/NCI NIH HHS/United States
- AI38910/AI/NIAID NIH HHS/United States
- R01 AI069152/AI/NIAID NIH HHS/United States
- R01 AI038910/AI/NIAID NIH HHS/United States
- AI069152/AI/NIAID NIH HHS/United States
- R29 AI038910/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous