Single-molecule force spectroscopy distinguishes target binding modes of calmodulin - PubMed (original) (raw)
Single-molecule force spectroscopy distinguishes target binding modes of calmodulin
Jan Philipp Junker et al. Proc Natl Acad Sci U S A. 2009.
Abstract
The eukaryotic signaling protein calmodulin (CaM) can bind to more than 300 known target proteins to regulate numerous functions in our body in a calcium-dependent manner. How CaM distinguishes between these various targets is still largely unknown. Here, we investigate fluctuations of the complex formation of CaM and its target peptide sequences using single-molecule force spectroscopy by AFM. By applying mechanical force, we can steer a single CaM molecule through its folding energy landscape from the fully unfolded state to the native target-bound state revealing equilibrium fluctuations between numerous intermediate states. We find that the prototypical CaM target sequence skMLCK, a fragment from skeletal muscle myosin light chain kinase, binds to CaM in a highly cooperative way, while only a lower degree of interdomain binding cooperativity emerges for CaMKK, a target peptide from CaM-dependent kinase kinase. We identify minimal binding motifs for both of these peptides, confirming that affinities of target peptides are not exclusively determined by their pattern of hydrophobic anchor residues. Our results reveal an association mode for CaMKK in which the peptide binds strongly to only partially Ca(2+)-saturated CaM. This binding mode might allow for a fine-tuning of the intracellular response to changes in Ca(2+) concentration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
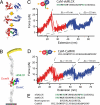
Fig. 1.
(A) Structure of CaM in different ligand binding states. DomN is shown in red, DomC in blue. Upper picture, apo CaM; middle, Ca2+ loaded form, Ca2+ ions are shown in gray; lower picture, Ca2+-CaM bound to target peptide skMLCK (green). (B) Scheme of the experimental setup. CaM-skMLCK is incorporated into filamin domains (gray) that serve as handles for attaching the protein construct to a surface and to an AFM cantilever tip. (C) Typical force vs. extension trace of skMLCK fused to CaM (CaM-skMLCK) at a pulling velocity of vpull = 0.5 nm/s. Unfolding peaks of DomN and DomC of CaM-skMLCK are shown in red and blue, respectively. WLC curves are shown in gray. In the skMLCK amino acid sequence, hydrophobic anchor residues are highlighted in bold type, charged amino acids directly adjacent to these hydrophobic residues are colored in green. (D) Force vs. extension trace of CaMKK inserted between DomN and DomC of CaM (CaM-CaMKK), recorded at a pulling velocity of 1 nm/s. Again, unfolding peaks of DomN and DomC of CaM-skMLCK are shown in red and blue, respectively. (E) Sequence alignment of the target peptides skMLCK, smMLCK, mastoparan, and CaMKK. Hydrophobic anchor residues are shown in bold font, and charged amino acids directly adjacent to hydrophobic anchor residues are colored in green.
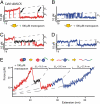
Fig. 2.
(A and B) Time traces of the unfolding regions of DomN (A) and DomC (B) of CaM-skMLCK, vpull = 0.5 nm/s. A short-lived intermediate level appears for DomN. No such level can be distinguished in DomC. (C and D) Time traces of DomN (C) and DomC (D) of CaM-skMLCK in the presence of 100 μM mastoparan in solution, vpull = 1 nm/s. Under these conditions, the intermediate level of DomN (shown in black) is stabilized and the transition kinetics is slowed down. The intermediate level can be found in all traces, suggesting an obligatory intermediate. (E) Force vs. extension trace of CaM-skMLCK at 100 μM mastoparan, vpull = 10 nm/s, and WLC fit curves (dashed gray lines). The sequence of the structural transitions of CaM-skMLCK under force (scheme above the trace) can be reconstructed from the length gains of the individual unfolding events.
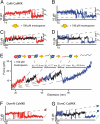
Fig. 3.
(A and B) Time traces of the unfolding regions of DomN (A) and DomC (B) of CaM-CaMKK, vpull = 1 nm/s. For both domains, a short-lived intermediate level appears. (C and D) Time traces of DomN (C) and DomC (D) of CaM-CaMKK at 100 μM mastoparan, vpull = 1 nm/s. The intermediate state (shown in black) is stabilized and can now be clearly distinguished from the two other levels. (E) Force vs. extension trace of CaM-CaMKK at 100 μM mastoparan, vpull = 10 nm/s and WLC fit curves (dashed gray lines). Above the trace, we show a scheme of the sequence of structural transitions as inferred from the increases in contour length. (F) Time trace of isolated DomN fused to CaMKK (DomN-CaMKK) at 10 μM mastoparan. No intermediate level can be detected, hence no sufficiently strong binding of CaMKK to isolated DomN takes place. (G) Time trace of isolated DomC fused to CaMKK (DomC-CaMKK) at 10 μM mastoparan, showing three levels (DomC folded, peptide-bound; DomC folded, peptide-unbound; DomC unfolded).
Fig. 4.
(A and B) Time traces of the transition region of DomN (A) and DomC (B) of the CaM-skMLCK (–18) truncation mutant at 100 μM mastoparan, vpull = 1 nm/s. As in the case of full-length CaM-skMLCK, an intermediate level appears for DomN (shown in black), but not for DomC. In the amino acid sequence of the target peptide, residues that have been removed are colored in gray and light green. (C) Time trace of DomC-CaMKK (–26) at 10 μM mastoparan, vpull = 1 nm/s. This truncated version of CaMKK is sufficient for strong binding to DomC. (D) Time trace of DomC-CaMKK (–26) at 10 μM mastoparan, vpull = 1 nm/s. No intermediate level can be resolved, suggesting residues 9–14 of CaMKK are important for high-affinity binding to DomC.
Fig. 5.
(A) Pulling speed dependence of mean unbinding force (first peak of the trace) of skMLCK (1–26; dark green) and skMLCK (1–18; light green) at nonequilibrium conditions. Results of Monte Carlo simulations are shown in black. (B) Calculated potential energy landscape at zero force for binding/unbinding of skMLCK (–26) and skMLCK (–18) to CaM. (C) Unbinding forces vs. pulling speed are shown for CaMKK N (first peak in Fig. 3_E_) and CaMKK C (third peak in Fig. 3_E_) in red and blue, respectively. Since equilibrium conditions prevail at low pulling velocities, forces of the first unbinding transition were analyzed. Results of Monte Carlo simulations are shown in black. (D) Calculated potential energy landscape at zero force for binding/unbinding of CaMKK N and CaMKK C.
Similar articles
- Ligand-dependent equilibrium fluctuations of single calmodulin molecules.
Junker JP, Ziegler F, Rief M. Junker JP, et al. Science. 2009 Jan 30;323(5914):633-7. doi: 10.1126/science.1166191. Science. 2009. PMID: 19179531 - Variable conformation and dynamics of calmodulin complexed with peptides derived from the autoinhibitory domains of target proteins.
Yao Y, Squier TC. Yao Y, et al. Biochemistry. 1996 May 28;35(21):6815-27. doi: 10.1021/bi960229k. Biochemistry. 1996. PMID: 8639633 - Further insights into calmodulin-myosin light chain kinase interaction from solution scattering and shape restoration.
Heller WT, Krueger JK, Trewhella J. Heller WT, et al. Biochemistry. 2003 Sep 16;42(36):10579-88. doi: 10.1021/bi0348664. Biochemistry. 2003. PMID: 12962481 - Structure, dynamics and interaction with kinase targets: computer simulations of calmodulin.
Yang C, Jas GS, Kuczera K. Yang C, et al. Biochim Biophys Acta. 2004 Mar 11;1697(1-2):289-300. doi: 10.1016/j.bbapap.2003.11.032. Biochim Biophys Acta. 2004. PMID: 15023369 Review. - Structural diversity of calmodulin binding to its target sites.
Tidow H, Nissen P. Tidow H, et al. FEBS J. 2013 Nov;280(21):5551-65. doi: 10.1111/febs.12296. Epub 2013 May 13. FEBS J. 2013. PMID: 23601118 Review.
Cited by
- Tracking unfolding and refolding reactions of single proteins using atomic force microscopy methods.
Bujalowski PJ, Oberhauser AF. Bujalowski PJ, et al. Methods. 2013 Apr 1;60(2):151-60. doi: 10.1016/j.ymeth.2013.03.010. Epub 2013 Mar 20. Methods. 2013. PMID: 23523554 Free PMC article. - Further Evidence of the Melatonin Calmodulin Interaction: Effect on CaMKII Activity.
Argueta J, Solís-Chagoyán H, Estrada-Reyes R, Constantino-Jonapa LA, Oikawa-Sala J, Velázquez-Moctezuma J, Benítez-King G. Argueta J, et al. Int J Mol Sci. 2022 Feb 24;23(5):2479. doi: 10.3390/ijms23052479. Int J Mol Sci. 2022. PMID: 35269623 Free PMC article. - The effect of macromolecular crowding, ionic strength and calcium binding on calmodulin dynamics.
Wang Q, Liang KC, Czader A, Waxham MN, Cheung MS. Wang Q, et al. PLoS Comput Biol. 2011 Jul;7(7):e1002114. doi: 10.1371/journal.pcbi.1002114. Epub 2011 Jul 28. PLoS Comput Biol. 2011. PMID: 21829336 Free PMC article. - Manipulating protein conformations by single-molecule AFM-FRET nanoscopy.
He Y, Lu M, Cao J, Lu HP. He Y, et al. ACS Nano. 2012 Feb 28;6(2):1221-9. doi: 10.1021/nn2038669. Epub 2012 Feb 1. ACS Nano. 2012. PMID: 22276737 Free PMC article. - Unraveling the Mechanical Unfolding Pathways of a Multidomain Protein: Phosphoglycerate Kinase.
Li Q, Scholl ZN, Marszalek PE. Li Q, et al. Biophys J. 2018 Jul 3;115(1):46-58. doi: 10.1016/j.bpj.2018.05.028. Biophys J. 2018. PMID: 29972811 Free PMC article.
References
- Chin D, Means AR. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. - PubMed
- Chen YG, Hummer G. Slow conformational dynamics and unfolding of the calmodulin C-terminal domain. J Am Chem Soc. 2007;129:2414–2415. - PubMed
- Yamniuk AP, Vogel HJ. Calmodulin's flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol Biotechnol. 2004;27:33–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous