Immortalization eliminates a roadblock during cellular reprogramming into iPS cells - PubMed (original) (raw)
. 2009 Aug 27;460(7259):1145-8.
doi: 10.1038/nature08285. Epub 2009 Aug 9.
Affiliations
- PMID: 19668190
- PMCID: PMC3987892
- DOI: 10.1038/nature08285
Immortalization eliminates a roadblock during cellular reprogramming into iPS cells
Jochen Utikal et al. Nature. 2009.
Abstract
The overexpression of defined transcription factors in somatic cells results in their reprogramming into induced pluripotent stem (iPS) cells. The extremely low efficiency and slow kinetics of in vitro reprogramming suggest that further rare events are required to generate iPS cells. The nature and identity of these events, however, remain elusive. We noticed that the reprogramming potential of primary murine fibroblasts into iPS cells decreases after serial passaging and the concomitant onset of senescence. Consistent with the notion that loss of replicative potential provides a barrier for reprogramming, here we show that cells with low endogenous p19(Arf) (encoded by the Ink4a/Arf locus, also known as Cdkn2a locus) protein levels and immortal fibroblasts deficient in components of the Arf-Trp53 pathway yield iPS cell colonies with up to threefold faster kinetics and at a significantly higher efficiency than wild-type cells, endowing almost every somatic cell with the potential to form iPS cells. Notably, the acute genetic ablation of Trp53 (also known as p53) in cellular subpopulations that normally fail to reprogram rescues their ability to produce iPS cells. Our results show that the acquisition of immortality is a crucial and rate-limiting step towards the establishment of a pluripotent state in somatic cells and underscore the similarities between induced pluripotency and tumorigenesis.
Figures
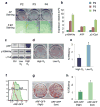
Figure 1. Reprogramming efficiency of fibroblasts is influenced by replicative potential and ARF expression status
(a) Alkaline phosphatase (AP) staining (top) of iPS cell colonies derived from secondary murine embryonic fibroblasts (MEFs) at different passages (P). Senescence associated β-galactosidase activity (bottom) of MEFs at same passages. (b) Expression levels of p16INK4a, ARF and p21Cip1 in MEFs at the same passages as shown in (a). (c) Western blot analysis for p16INK4a and phospho-p53 (p53*) in MEFs grown at low (4%) or high (21 %) oxygen. (d, e) Secondary MEFs grown under low O2 give rise to iPS cells more efficiently. (f) ARF-GFP reporter MEFs (green line) at passage 3 show heterogeneous expression levels. Shown in red are wild type (WT) MEFs. (g, h) ARF-GFPlow MEFs give rise to transgene-independent AP+ iPS colonies more efficiently than ARF-GFPhigh cells. Error bars depict the s.e.m.
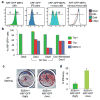
Figure 2. Transcription factor-induced downregulation of INK4a/ARF expression in cells undergoing reprogramming
(a) FACS plots of sorted ARF-GFPhigh MEFs (first panel), established iPS cells from ARF-GFP MEFs (second panel), ARF-GFPhigh MEFs expressing all four reprogramming factors (third panel) or each factor individually (last panel). (b) Time course of ARF-GFP expression in subpopulations of cells undergoing reprogramming. (c, d) ARF-GFPlow SSEA1+ cells at 6 days of transgene expression give rise to more transgene-independent AP+ iPS cell colonies than ARF-GFPhigh SSEA1+ cells. Error bars depict the s.e.m.
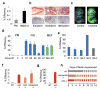
Figure 3. Cellular immortalization enhances reprogramming potential and kinetics
(a) Spontaneously immortalized Melan A cell line yields iPS colonies 3–4 times more efficiently than primary melanocytes (PM) upon direct viral infection. (b, c) Melan A-drived iPS cells show differentiation into ectodermal, mesodermal and endodermal derivatives in teratomas (b, top panel) and in chimeras produced from iPS cells labeled with a lentivirus constitutively expressing GFP (b, bottom panel, and c). (d) iPS cell formation efficiency of secondary (2°) cells derived from PMs (grey bars), Melan A-derived _in vitro_-differentiated (IVD) cells (blue bars) or Melan A-derived MEFs (green bars). (e) iPS cell formation efficiency of subclones of Melan A-derived IVD 2° cells. (f) Reprogramming efficiency of WT, p53−/−, ARF−/− and INK4a/ARF−/− MEFs upon direct viral infection. (g) Reprogramming potential of 2° p53−/− iPS cell-derived E14.5 MEFs. (h) Evaluation of minimal temporal transgene requirement (solid lines) in WT, INK4a/ARF−/− and p53−/− MEFs to form stable iPS cell colonies.
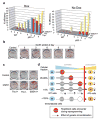
Figure 4. p53 deficiency rescues reprogramming potential in cells that normally fail to form iPS cells
(a) Comparison of reprogramming potentials of sorted Thy1+, Thy1− and SSEA1+ subpopultions in WT and p53−/− cells at different time points during reprogramming in the presence or absence of doxycycline (dox). (b) Acute inactivation of p53 by lentivirus expressing short hairpin (shp53) in secondary cells increases reprogramming efficiency at all time points. (c) Knockdown of p53 by shp53 recues potential of Thy1− and Thy1+ subpopulations to generate iPS cells. (d) Model summarizing the presented data; during factor-induced reprogramming, cells encounter different roadblocks such as the succesful silencing of somatics genes (e.g., Thy1), the activation of pluripotency genes (e.g., SSEA1) and eventually the acquisition of immortality (e.g., silencing of ARF). The low efficiency of the process is likely due to the capacity of rare cells to overcome these roadblocks. In immortal fibroblasts, however, almost every cell is endowed with the potential to produce iPS cells. Moreover, cells that have already encountered a roadblock can be rescued by acute inactivation of p53 (indicated by dashed black lines). Red bar illustrates the transition point between somatic (blue) and pluripotent (yellow) state.
Comment in
- Stem cells: The promises and perils of p53.
Krizhanovsky V, Lowe SW. Krizhanovsky V, et al. Nature. 2009 Aug 27;460(7259):1085-6. doi: 10.1038/4601085a. Nature. 2009. PMID: 19713919 Free PMC article.
Similar articles
- The Ink4/Arf locus is a barrier for iPS cell reprogramming.
Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, Blasco MA, Serrano M. Li H, et al. Nature. 2009 Aug 27;460(7259):1136-9. doi: 10.1038/nature08290. Epub 2009 Aug 9. Nature. 2009. PMID: 19668188 Free PMC article. - Linking the p53 tumour suppressor pathway to somatic cell reprogramming.
Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisúa Belmonte JC. Kawamura T, et al. Nature. 2009 Aug 27;460(7259):1140-4. doi: 10.1038/nature08311. Epub 2009 Aug 9. Nature. 2009. PMID: 19668186 Free PMC article. - Stem cells: The promises and perils of p53.
Krizhanovsky V, Lowe SW. Krizhanovsky V, et al. Nature. 2009 Aug 27;460(7259):1085-6. doi: 10.1038/4601085a. Nature. 2009. PMID: 19713919 Free PMC article. - Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming.
Ong ALC, Ramasamy TS. Ong ALC, et al. Ageing Res Rev. 2018 May;43:64-80. doi: 10.1016/j.arr.2018.02.004. Epub 2018 Feb 21. Ageing Res Rev. 2018. PMID: 29476819 Review. - How to become immortal: let MEFs count the ways.
Odell A, Askham J, Whibley C, Hollstein M. Odell A, et al. Aging (Albany NY). 2010 Mar 31;2(3):160-5. doi: 10.18632/aging.100129. Aging (Albany NY). 2010. PMID: 20378935 Free PMC article. Review.
Cited by
- The emerging functions of the p53-miRNA network in stem cell biology.
Lin CP, Choi YJ, Hicks GG, He L. Lin CP, et al. Cell Cycle. 2012 Jun 1;11(11):2063-72. doi: 10.4161/cc.20207. Epub 2012 Jun 1. Cell Cycle. 2012. PMID: 22580472 Free PMC article. Review. - Regulation of pluripotency and self- renewal of ESCs through epigenetic-threshold modulation and mRNA pruning.
Jia J, Zheng X, Hu G, Cui K, Zhang J, Zhang A, Jiang H, Lu B, Yates J 3rd, Liu C, Zhao K, Zheng Y. Jia J, et al. Cell. 2012 Oct 26;151(3):576-89. doi: 10.1016/j.cell.2012.09.023. Cell. 2012. PMID: 23101626 Free PMC article. - p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation.
Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Cañamero M, Rizzoti K, Carneiro C, Martínez G, Vidal A, Lovell-Badge R, Serrano M. Li H, et al. Cell Stem Cell. 2012 Dec 7;11(6):845-52. doi: 10.1016/j.stem.2012.09.014. Cell Stem Cell. 2012. PMID: 23217425 Free PMC article. - Nerve growth factor receptor negates the tumor suppressor p53 as a feedback regulator.
Zhou X, Hao Q, Liao P, Luo S, Zhang M, Hu G, Liu H, Zhang Y, Cao B, Baddoo M, Flemington EK, Zeng SX, Lu H. Zhou X, et al. Elife. 2016 Jun 10;5:e15099. doi: 10.7554/eLife.15099. Elife. 2016. PMID: 27282385 Free PMC article. - Generation of an inducible fibroblast cell line for studying direct cardiac reprogramming.
Vaseghi HR, Yin C, Zhou Y, Wang L, Liu J, Qian L. Vaseghi HR, et al. Genesis. 2016 Jul;54(7):398-406. doi: 10.1002/dvg.22947. Epub 2016 Jun 1. Genesis. 2016. PMID: 27194122 Free PMC article.
References
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. - PubMed
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
- Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. - PubMed
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous