A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth - PubMed (original) (raw)
A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth
Binzhi Qian et al. PLoS One. 2009.
Abstract
Background: The stromal microenvironment and particularly the macrophage component of primary tumors influence their malignant potential. However, at the metastatic site the role of these cells and their mechanism of actions for establishment and growth of metastases remain largely unknown.
Methodology/principal findings: Using animal models of breast cancer metastasis, we show that a population of host macrophages displaying a distinct phenotype is recruited to extravasating pulmonary metastatic cells regardless of species of origin. Ablation of this macrophage population through three independent means (genetic and chemical) showed that these macrophages are required for efficient metastatic seeding and growth. Importantly, even after metastatic growth is established, ablation of this macrophage population inhibited subsequent growth. Furthermore, imaging of intact lungs revealed that macrophages are required for efficient tumor cell extravasation.
Conclusion/significance: These data indicate a direct enhancement of metastatic growth by macrophages through their effects on tumor cell extravasation, survival and subsequent growth and identifies these cells as a new therapeutic target for treatment of metastatic disease.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
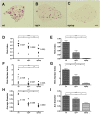
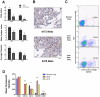
Figure 1. Host CSF-1 determines tumor cell pulmonary seeding and persistent growth.
(A–C) Different metastatic potential of primary PyMT induced tumor cells shown in H&E stained transverse section of host lungs of different mouse genotypes. Wild type (wt), heterozygous Csf1op (op/+) and homozygous Csf1op (op/op) (D–I) Quantifications of metastasis of primary PyMT tumor cells (D, F and H) and Met-1 cells (E, G and I) using stereological methods. Metastasis index (D and E) is equal to total metastasis volume normalized by total lung volume (note log scale on Y axis in D); Metastasis number index (F and G) is equal to averaged number of metastasis sites per mm2 lung area; Average diameter (H and I) is the averaged size of metastasis nodules in millimeter. For primary PyMT cells n = 7, in each graph, data points in mice of different genotypes use the same symbol for each individual tumor. For Met-1 cells data are shown as mean+SEM. n≥4, *p<0.05, **P<0.01 and ***p<0.001.
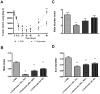
Figure 2. Macrophage depletion blocks tumor cell pulmonary seeding and persistent growth.
(A) Absolute number of surviving tumor cells in the lung at different time points indicated after the tail vein injection (n≥3, *p<0.05). Open box, mice treated with liposome-PBS; solid triangle, mice treated with liposome-Clodronate. Data are shown as mean±SEM. (B–D) In vivo macrophage depletion blocks tumor cell pulmonary seeding and persistent growth. In-house prepared liposome-Clodronate was injected i.v. into the tail veins of mice at time indicated according to the time of Met-1 cell injection to deplete macrophage in vivo. Metastasis quantification was the same as in Fig. 1. n≥5, **P<0.01.
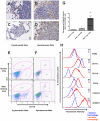
Figure 3. Recruitment of a distinct macrophage population in metastasis bearing lungs.
(A–D) Pulmonary metastasis of breast cancer cells are highly infiltrated with macrophages. Representative Mac3 immunohistochemistry staining of transverse sections of lung metastatic lesions from different tumor models: (A) experimental metastasis of primary PyMT tumor cells; (B) spontaneous metastasis derived from a MMTV-PyMT induced mammary tumor; (C) experimental metastasis of Met-1 cells and (D) spontaneous metastasis derived from subcutaneously implanted MDA-231 cells. Bar equals 20 um. (E) Representative flow diagram of CSF-1R-GFP positive cells from normal lung (upper panel) and metastasis bearing lung from experimental metastasis assay of Met-1 cells (lower panel). n = 5 (F) Representative flow diagram of CSF-1R-GFP positive cells from normal lung (upper panel) and lung bearing spontaneous metastasis from MMTV-PyMT induced mouse mammary tumor (lower panel). n = 3 (G) Recruitment of CD11b+Gr1- macrophages (F4/80+) in lungs with experimentally induced metastasis with Met-1 cells. Lungs were harvested at time indicated after tumor cell i.v. injection. Data are shown as mean+SEM. n = 3, *p<0.05 and **P<0.01. (H) Representative flow histograms of normal lung macrophages (F4/80+, blue dashed line) versus recruited macrophage population (F4/80+CD11b+Gr1-, red solid line) from lungs bearing Met-1 cell metastases stained with antibodies of different cell surface makers (indicated at the right side of the histogram). X axis indicates the fluorescent intensity, Y axis indicates the percentage of maximum cell number, MFI (top right panel) denotes representative mean fluorescent intensity (n = 3).
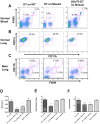
Figure 4. Ablation of CD11b+macrophages blocks tumor cell pulmonary seeding and persistent growth.
(A) Representative flow diagram of blood CD45+cells in mice treated with diphtheria toxin (DT) or Glu52 mutated DT. X axis, CD11b expression; Y axis, F4/80 expression. (B) Representative flow diagram of lung F4/80+cells in mice treated with DT or Glu52-DT. X axis, CD11b expression; Y axis, CD11c expression. (C) Representative flow diagram of lungs CD45+cells of mice with experimentally introduced Met-1 cell metastases before DT or Glu52-DT treatment. X axis, F4/80 expression; Y axis, CD11b expression. (D–F) In vivo depletion of CD11b+macrophage blocks tumor cell pulmonary seeding and persistent growth. DT was given i.p. at the times indicated according to Met-1 cell injection and metastasis quantifications were the same as in Fig. 1. DT treatment on mice with wild type bone marrow transplant and Glu52-DT treatment on mosaic mice were used as controls. Data are shown as mean+SEM. n≥5, *p<0.05 and **P<0.01.
Figure 5. Host CD11b+Gr1- macrophages promote human breast cancer cell experimental metastasis.
(A) In vivo macrophage depletion by liposome encapsulated Clodronate blocks tumor cell pulmonary seeding and persistent growth of MDA-231 derived human breast cancer cell lines, 3475 and 4173. Metastasis quantifications were the same as in Fig. 1. Data are shown as mean+SEM. n = 5, *p<0.05, **P<0.01 and ***p<0.001. (B) Pulmonary metastases of human breast cancer cells in nude mice are highly infiltrated with macrophages with anti-Mac3 antibody staining as described in methods. (C) Representative flow diagrams of CD11b+Gr1- cells recruitment by pulmonary metastases of 4173 and 3475. Lung F4/80+cells were separated by surface expression of CD11b (X axis) and Gr1 (Y axis), n = 3 (D) A graph comparing flow cytometric data of mean fluorescent intensity of different cell surface markers (F4/80, CD11b, CD11c, VEGFR1 and CCR2) expressed by normal lung macrophages (left histogram) and macrophages recruited by pulmonary metastases of 3475 (middle histogram) and 4173 (right histogram). Data are shown as mean+SEM. n = 3, *p<0.05, **P<0.01 and ***p<0.001.
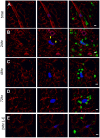
Figure 6. Tumor-macrophage interaction in the lung using ex vivo imaging.
Representative snapshots of 3D reconstructed confocal images of tumor cell (CFP, shown in blue) and macrophage (GFP, shown in green) at different times indicated after tumor cell tail vein injection: 5 minutes (A), 24 hours (B), 48 hours (C), 72 hours (D) and 24 hours in L-Clodronate treated mouse (E). Blood vessel were stained with Alexa Fluor® 647 conjugated anti-mouse CD31 antibody (shown in red). Bar equals 20 um. Arrow heads in B indicate the extravasated part of the tumor cell.
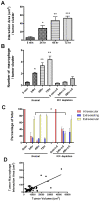
Figure 7. Tumor-macrophage interactions promotes tumor cell extravasation and correlates with initial tumor growth.
(A) Average area of direct interaction between tumor cells and macrophages per tumor cluster measured by confocal microscopy at different time points after iv injection. Statistically different from 5 min time point *p<0.05, **P<0.01 and ***p<0.001 (B) Left four histograms: Average number of macrophages that directly interact with each tumor cell cluster at different time points after iv injection. Statistically different from the 5 min control *p<0.05, **P<0.01. Right three histograms: Interactions following macrophage depletion using Liposome-Clodronate administered 16 hrs before and 2 hours after iv injection as described in the materials and methods. All time points are significantly lower than the macrophage replete mice. (C) Tumor cell extravasation status at different time points after iv injection showing the percentage of totally intravascular (red), extravasating (blue, inside vessels and outside) and extravascular (yellow, completely outside vessels) in normal or macrophage depleted mice using Liposome-Clodronate as described above. Note that data is represented as a percentage of total cells although the number of viable tumor cells is greatly reduced after macrophage depletion (Fig. 2A). The delay in extravasation at 24 hrs following macrophage depletion is statistically significant with p<0.05. (D) Correlation between tumor cluster volume and tumor-macrophage interaction area at 72 hours after tumor cell tail vein injection. p<0.0001, R2 = 0.58 (A–C are based upon 3D images of 10–20 clusters per animal, 3–6 mice per time point. Data are shown as mean+SEM. D., 51 tumor clusters from 3 mice).
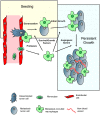
Figure 8. Model for macrophage promotion of metastasis at distant sites.
Based on the data in current study, we suggest a model for the macrophage enhancement of metastasis at the distal target organ. Following arrest of the tumor cells in capillaries of metastasis target organ, monocytes are quickly recruited and differentiate in situ into metastasis associated macrophage phenotype whereafter they promote the different steps of metastatic seeding, initial and persistent growth as indicated in the figure and described in the discussion. Figure modified from Joyce and Pollard, 2009.
Similar articles
- Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer.
Steenbrugge J, Breyne K, Demeyere K, De Wever O, Sanders NN, Van Den Broeck W, Colpaert C, Vermeulen P, Van Laere S, Meyer E. Steenbrugge J, et al. J Exp Clin Cancer Res. 2018 Aug 15;37(1):191. doi: 10.1186/s13046-018-0860-x. J Exp Clin Cancer Res. 2018. PMID: 30111338 Free PMC article. - Intravital imaging reveals distinct responses of depleting dynamic tumor-associated macrophage and dendritic cell subpopulations.
Lohela M, Casbon AJ, Olow A, Bonham L, Branstetter D, Weng N, Smith J, Werb Z. Lohela M, et al. Proc Natl Acad Sci U S A. 2014 Nov 25;111(47):E5086-95. doi: 10.1073/pnas.1419899111. Epub 2014 Nov 10. Proc Natl Acad Sci U S A. 2014. PMID: 25385645 Free PMC article. - Macrophages in cancer metastases and their relevance to metastatic growth.
Key ME. Key ME. Cancer Metastasis Rev. 1983;2(1):75-88. doi: 10.1007/BF00046906. Cancer Metastasis Rev. 1983. PMID: 6352008 Review. - Redefining macrophage and neutrophil biology in the metastatic cascade.
Güç E, Pollard JW. Güç E, et al. Immunity. 2021 May 11;54(5):885-902. doi: 10.1016/j.immuni.2021.03.022. Immunity. 2021. PMID: 33979586 Review.
Cited by
- A bi-directional dialog between vascular cells and monocytes/macrophages regulates tumor progression.
Delprat V, Michiels C. Delprat V, et al. Cancer Metastasis Rev. 2021 Jun;40(2):477-500. doi: 10.1007/s10555-021-09958-2. Epub 2021 Mar 30. Cancer Metastasis Rev. 2021. PMID: 33783686 Free PMC article. Review. - Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature.
Deryugina EI, Quigley JP. Deryugina EI, et al. Matrix Biol. 2015 May-Jul;44-46:94-112. doi: 10.1016/j.matbio.2015.04.004. Epub 2015 Apr 22. Matrix Biol. 2015. PMID: 25912949 Free PMC article. Review. - Influencing tumor-associated macrophages in malignant melanoma with monoclonal antibodies.
Adams R, Osborn G, Mukhia B, Laddach R, Willsmore Z, Chenoweth A, Geh JLC, MacKenzie Ross AD, Healy C, Barber L, Tsoka S, Sanz-Moreno V, Lacy KE, Karagiannis SN. Adams R, et al. Oncoimmunology. 2022 Oct 3;11(1):2127284. doi: 10.1080/2162402X.2022.2127284. eCollection 2022. Oncoimmunology. 2022. PMID: 36211808 Free PMC article. Review. - Intravital imaging.
Pittet MJ, Weissleder R. Pittet MJ, et al. Cell. 2011 Nov 23;147(5):983-91. doi: 10.1016/j.cell.2011.11.004. Cell. 2011. PMID: 22118457 Free PMC article. Review. - In vitro model of tumor cell extravasation.
Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL. Jeon JS, et al. PLoS One. 2013;8(2):e56910. doi: 10.1371/journal.pone.0056910. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437268 Free PMC article.
References
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
- Varghese HJ, Davidson MT, MacDonald IC, Wilson SM, Nadkarni KV, et al. Activated ras regulates the proliferation/apoptosis balance and early survival of developing micrometastases. Cancer Res. 2002;62:887–891. - PubMed
- Wong CW, Lee A, Shientag L, Yu J, Dong Y, et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61:333–338. - PubMed
- Fokas E, Engenhart-Cabillic R, Daniilidis K, Rose F, An HX. Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA 100324/CA/NCI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- P01 CA100324/CA/NCI NIH HHS/United States
- P30 CA 13330/CA/NCI NIH HHS/United States
- R01 CA 94173/CA/NCI NIH HHS/United States
- R01 CA094173/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases