Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila - PubMed (original) (raw)
Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila
Phil G T Sanders et al. PLoS Biol. 2009 Aug.
Abstract
Notch receptors act as ligand-dependent membrane-tethered transcription factors with a prominent role in binary cell fate decisions during development, which is conserved across species. In addition there is increasing evidence for other functions of Notch, particularly in connection with Wnt signalling: Notch is able to modulate the activity of Armadillo/ss-catenin, the effector of Wnt signalling, in a manner that is independent of its transcriptional activity. Here we explore the mechanism of this interaction in the epithelium of the Drosophila imaginal discs and find that it is mediated by the ligand-independent endocytosis and traffic of the Notch receptor. Our results show that Notch associates with Armadillo near the adherens junctions and that it is rapidly endocytosed promoting the traffic of an activated form of Armadillo into endosomal compartments, where it may be degraded. As Notch has the ability to interact with and downregulate activated forms of Armadillo, it is possible that in vivo Notch regulates the transcriptionally competent pool of Armadillo. These interactions reveal a previously unknown activity of Notch, which serves to buffer the function of activated Armadillo and might underlie some of its transcription-independent effects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Armadillo induces outgrowths in the absence of Notch.
Nomarski images of third instar wing discs. (A) Wild-type wing imaginal disc. (B) Wing disc expressing UAS_-ArmS10_ under the control of dpp_-Gal4. Notice the expansion of the hinge region (black arrow) and the associated deformation of the wing pouch. There is also a slight overgrowth in the scutellar region (white arrow). (C) Wing disc heterozygous for Notch, with FRT/FLP induced clones of Notch mutant cells expressing UAS_-ArmS10 under the control of _dpp_-Gal4. Notice the outgrowths with tumorous appearance (grey arrows) masking the normal features of the wing disc. All pictures taken at the same magnification.
Figure 2. Armadillo induces defects in cell proliferation and adhesion in the absence of Notch.
Confocal images of third instar wings discs with MARCM clones (labelled in green) of Notch mutant cells (A and A1), Notch mutant cells that overexpress ArmS10 (B and B1), and Notch mutant cells that overexpress ArmS10 and CeN, induced at 48–72 h (A–C) or 72–96 h AEL (A1–C1). The small insets at the corners of each image are low magnification pictures of the discs shown which act as a reference (see Figure S4 for larger images of these insets). In this and the following images of related experiments, the pictures on the top and the right represent optical z-sections through the clones following the green and the red lines shown in the main picture. Notice that the clones expressing ArmS10 are much larger and also show a rounded appearance with little or no contacts with the apical and basal regions of the epithelium. This is particularly clear in clones induced in the 48–72 h period. In some instances one can observe large clones in the peripodial membrane, which exhibit the unusual feature of fusing with clones generated in the disc epithelium. See Figures S2 and S3, and Videos S1 and S2, for more details of the outgrowths. The expression of CeN in the clones of Notch mutant cells that overexpress ArmS10 reduces the proliferation effect, and corrects the loss of basal connection and the adhesion defects. See Video S3 for the complete z-stack on the N55e11;UAS_-ArmS10,CeN_ clone shown in (C). The red channel shows Scribble (a basolateral cell junction marker) and the blue, the DCadherin staining (an adherens junction marker). All pictures taken at the same magnification. Scale bar in (C1), 20 µm.
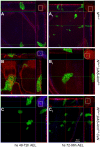
Figure 3. Absence of the Notch ligands Delta and Serrate or the transcriptional effector Su(H) does not affect the activity of Armadillo.
Confocal images of third instar wings discs with clones of cells induced at 48–72-h AEL mutant for both Dlrev10 and SerRx82 (A and B) or Su(H) Δ_47_ (C and D), without and with ArmS10 as indicated. Clones of cells mutant for Dlrev10 and SerRx82 (A) grow and remain integrated in the epithelium maintaining their apicobasal polarity as can be seen in the optical z-section. No major changes are observed when ArmS10 is expressed in these cells (B). Clones of cells mutant for Su(H) Δ_47_ display a number of distinctive features ([C] and see Video S4). They are smaller and appear more dispersed than those of cells mutant for Dlrev10 and SerRx82. The edges of the clones are ragged and irregular and give the impression that the cells are dispersing. Most notably cells can be observed dispersed within the plane of the epithelium with a large amount of apoptotic cells on the basal side (white arrow, see optical z-section). Expression of ArmS10 in these cells increases the size of the clones, alters their appearance, and reduces, but does not eliminate, the number of apoptotic cells in the basal region (see Video S5). Technical details of the images as in Figure 2 with the small insets at the corners of each image being low magnification pictures of the discs shown which act as a reference. Scale bar in (D), 20 µm.
Figure 4. Localization of CeN, CN, and Ce in the epithelial cells of third instar wing discs.
(A–A3) Localization of CeN at and above the adherens junctions (insets in A and A1 show the adherens junctions as labelled by DCad staining in the same image) as well as in more basal dots, which represent vesicles (A2–A3). The localization is revealed by the fluorescence of the eGFP. Notice that there is a pool of Notch apical to the adherens junctions. (B–B2) Localization of Ce in all membranes at all levels of the cell (B, apical' B1, subapical; B2, basal). This molecule contains the eGFP fused to the transmembrane and extracellular domains of CD8 and indicates that the localization of CeN is determined by the sequences of the Notch protein that are added to Ce. (C–E) Confocal optical z-sections through the wing pouch of a disc expressing CeN, showing the large vesicles of stain (C); CN, a chimera like CeN but without the eGFP that can only be visualized with an antibody against the intracellular domain of Notch, NICD, (D); and Ce, highlighting the overall and nonspecific distribution of eGFP to all membranes of the cell (E). Notice that in (D) it is not easy to distinguish the endogenous Notch from CN other than by the amount. The arrows in (C) and (E) point to the approximate levels of the pictures (A–A3) and (B–B2). The expression of the different proteins is directed by _dpp_-Gal4 in all cases. Scale bar, 10 µm. (F) Schematic of the structure of the Notch receptor and related chimeras used in this work.
Figure 5. Complementation of the buffering activity of Notch.
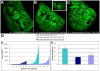
(A–C) Third instar imaginal wing discs containing Notch mutant clones (generated using the FRT/FLP system, labelled by the absence of GFP) and expressing UAS_-ArmS10_ alone (A) or in combination with UAS_-CeN_ (B) or UAS_-FLN_ (Full-Length-Notch) (C) under the control of _dpp_-Gal4 (see Materials and Methods for details). Notice that the clones are smaller and more frequent in (B) and (C) than those in (A). This mirrors the effects of the MARCM clones but because in these experiments the clones are induced continuously there are more clones that are averaged over the whole of imaginal development. The wing pouch of (C) tends to show a bigger size, which is due to the effect of the Su(H)-dependent activity of Notch, which is provided by FLN, and which cannot be provided by CeN . The dotted lines indicate the domain of the wing pouch analyzed in (D). The inset in (B) shows an optical z-section of the disc; the arrow points to CeN dots. (D) Quantification of the area of the clones in the wing pouch in the different genetic backgrounds (for details see Materials and Methods). At least three discs were analyzed per genotype. (D1) Distribution of the area and number of individual clones per domain. Every bar represents an individual clone with the size, expressed in pixels, indicated in the _y_-axis. The difference in numbers of clones is related to the number of experimental discs included in the count. Note that CeN and FLN reduce the area (size) of the clones. (D2) Relative average size of the clones analyzed in (D1).
Figure 6. Endocytosis and traffic of CeN reflects Notch.
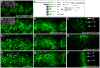
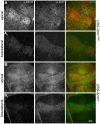
Expression of endogenous Notch in a wing disc in which UAS_-CeN_ is expressed under the control of _dpp_-Gal4. (A) Discs were fixed and permeabilized before staining with an antibody against the extracellular domain of Notch (red channel) and CD8 (blue channel). Optical sections through the apical (A) and the basal (A1) side of the cell. In apical levels, eGFP (green channel) and CD8 localize mostly in the membranes at the level of the adherens junctions (see also Figure 4A). Note that both in apical and basal levels the CD8 and eGFP vesicles colocalize. (B–D) Notch and CD8 tracked over time, by pulsing CeN expressing live wing discs with an antibody against the extracellular domain of Notch (red channel) and CD8 (blue channel), and chasing for 0 (B), 30 (C), and 60 min (D). (B–D, apical; B1–D1, basal sections). After 0 min of chasing, the endogenous Notch and CD8 localize in the apical membrane of the cells (B), and there are no vesicles in basal levels (B1). After 30 min of chase, the endogenous Notch has been cleared completely from the apical membranes and localizes in vesicles mostly in apical levels; at this time point, CD8 also goes to vesicles in the apical level, although some remain in the membrane (C–C1). After 60 min of chase, the endogenous Notch localizes in vesicles in apical and basal levels; at this time point, CD8 also goes to vesicles in both levels (D–D1). Circles mark some of the NECD, CeN, and CD8 colocalizing vesicles. In all cases the apical and basal images were taken in equivalent levels in the dorsal region of the wing pouch. The apical sections were taken at the level of the adherens junctions and the basal 7 µm underneath. See Figure S10 for an extended version of this figure. Scale bar, 10 µm.
Figure 7. Colocalization of Notch and Arm in endocytic vesicles.
(A) Notch and Armadillo colocalize at the apical membrane and in basally located vesicles. Image of a third instar wing disc of Arm-GFP flies in the region of the wing margin, fixed and stained for Arm (red channel), and NICD (blue channel). (A) Apical confocal section, at the level of the adherens junctions. (B) Basal section, 7 µm underneath the apical section. There is a fair amount of colocalization and a preliminary analysis of the colocalizing vesicles indicates that there are two types: those in which there is more Arm than NICD staining (marked with squares in the figure), and those that seemed to have more Notch than Arm (diamonds in the figure). Scale bar, 10 µm. (C–D) Results from an anti-Notch antibody loading and chase in wing discs expressing FLN under the control of _dpp_-Gal4. The antibody is against the extracellular domain of Notch (red channel) chasing for 10 min (C), and 30 min (D). The same discs were stained for Arm (green channel) after fixation. Note that there are Notch vesicles colocalizing with Arm, suggesting that both molecules traffic together. Subapical sections from the dorsal wing pouch are shown. Circles mark some of the NECD, and Arm colocalizing vesicles. Scale bar, 10 µm.
Figure 8. Notch recruits Armadillo to an apical domain in epithelial cells.
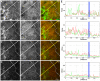
(A–D) Sequence of confocal sections (1 µm apart) from a third instar wing disc expressing FLN in the dpp domain of expression (located left of the dashed line; for details see Materials and Methods). Note that overexpression of FLN promotes the expansion of the cell surface–located Notch as revealed by anti-NICD antibody (green channel) to two sections (A and B) spanning at least 2 µm, rather than the single one in the adjacent wild-type cells. This expansion is mirrored in the localization of Armadillo (red channel), confirming the interaction between Notch and Armadillo and establishing the fact that FLN is able to recruit endogenous Armadillo to its specific apical domain. In (C and D) it is possible to observe an accumulation of Armadillo (both diffuse and in vesicles) also correlated with the presence of FLN. Scale bar, 10 µm. (E) Fluorescence intensity profiles of α-NICD (green line) and α-Arm staining (red line) along the yellow line in the (A–D) confocal sections. The blue line shows AP boundary (for details see Materials and Methods). Note that there is a clear increase in the Arm levels where there is overexpression of FLN (left from the blue line), that is more obvious in the two first (apical) sections.
Figure 9. Schematic summary of the reciprocal effects of Notch and Arm on their relative subcellular localization.
Each set of drawings represents a transversal section through the central region of a wing disc, in which cells expressing the indicated constructs are in yellow (wild-type ones are shown in white). The localization of the Myc-tag (from the ArmS10 molecule) is in red; NICD, in green; and the endogenous Arm in blue. See legends of Figures 8 and S13 for details. Expression of FLN shows that Notch can recruit Arm to its apical domain and also create a subapical domain where both can be found, sometimes, in vesicles. ArmS10 displaces endogenous Arm from the adherens junctions into a subapical domain, which is probably due to the increased stability of ArmS10. Expression of FLN or CeN together with ArmS10 leads to changes in the localization of Notch, Arm, and ArmS10 as shown. Overexpression of FLN shows that Notch can interact with Armadillo, which is in agreement with previous observations . On the other hand, when FLN is overexpressed with ArmS10, it induces changes in the localization of ArmS10 and Arm. While it is likely that Notch can interact with all forms of Arm, it is also possible that it interacts preferentially with ArmS10 and that the effects that we observe on Arm under these conditions are the result of the interactions with ArmS10. The observation that in the presence of FLN and ArmS10, Arm can be observed at the adherens junctions favours this possibility. NB: Most of the effects that we observe are restricted to the apical and subapical domains of the epithelial cells and it is important to bear in mind that it is not easy to discern much structure in this domain at the level of light microscopy as this appears to be the location of early, mid, late, and recycling endosomes.
Figure 10. Membrane tethered activated Arm induces relocalization of Notch in epithelial cells.
Analysis of the distribution and localization of Notch, monitored with a NICD antibody (in red), and endogenous Arm (in green) in wing discs expressing a myristylated N-terminal deleted Armadillo, ArmΔNMyr (A), or the same mutant without the myristylated signal, ArmΔN (B), under the control of _dpp_-Gal4. Confocal sections of the wing pouch are shown at the level of the adherens junctions (A and B) and a more basolateral (A1 and B1) region. The expression of ArmΔNMyr promotes an obvious accumulation of endogenous Notch in the apical (A) and basolateral (A1) membranes of the cells. The effect is very mild, though still visible, in the case of ArmΔN. Both forms of Arm also cause an effect in the endogenous Arm: it is weakly displaced from the apical membrane and accumulates in the cells, more obvious in ArmΔN (B1, for details of the distribution, effects, and interactions of these mutants with endogenous Armadillo, see [70]). Scale bar, 20 µm.
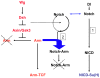
Figure 11. Mechanism for the buffering activity of Notch on Armadillo.
In the absence of Wingless (Wg), Axin/Gsk3-based destruction complex degrades Armadillo. In the absence of Wg, Dsh inhibits the complex, and Arm can enter the nucleus. We postulate that Notch is endocytosed through two different routes, one ligand dependent, which leads to the generation of NICD and Su(H)-dependent signalling and the other, ligand independent (2), which leads to degradation. In the ligand-independent route Notch associates with Armadillo/ß-catenin and directs it to degradation. This ligand independent activity of Notch would degrade the Arm that escapes from the Axin-mediated degradation.
Similar articles
- Modulation of the ligand-independent traffic of Notch by Axin and Apc contributes to the activation of Armadillo in Drosophila.
Muñoz-Descalzo S, Tkocz K, Balayo T, Arias AM. Muñoz-Descalzo S, et al. Development. 2011 Apr;138(8):1501-6. doi: 10.1242/dev.061309. Epub 2011 Mar 9. Development. 2011. PMID: 21389052 Free PMC article. - Notch synergizes with axin to regulate the activity of armadillo in Drosophila.
Hayward P, Balayo T, Martinez Arias A. Hayward P, et al. Dev Dyn. 2006 Oct;235(10):2656-66. doi: 10.1002/dvdy.20902. Dev Dyn. 2006. PMID: 16881048 - Kinase active Misshapen regulates Notch signaling in Drosophila melanogaster.
Mishra AK, Sachan N, Mutsuddi M, Mukherjee A. Mishra AK, et al. Exp Cell Res. 2015 Nov 15;339(1):51-60. doi: 10.1016/j.yexcr.2015.09.021. Epub 2015 Oct 9. Exp Cell Res. 2015. PMID: 26431585 - Letting go: modification of cell adhesion during apoptosis.
Suzanne M, Steller H. Suzanne M, et al. J Biol. 2009;8(5):49. doi: 10.1186/jbiol152. Epub 2009 May 28. J Biol. 2009. PMID: 19519938 Free PMC article. Review. - Endocytosis and control of Notch signaling.
Kandachar V, Roegiers F. Kandachar V, et al. Curr Opin Cell Biol. 2012 Aug;24(4):534-40. doi: 10.1016/j.ceb.2012.06.006. Epub 2012 Jul 18. Curr Opin Cell Biol. 2012. PMID: 22818956 Free PMC article. Review.
Cited by
- Inhibition of Wnt signalling by Notch via two distinct mechanisms.
Acar A, Hidalgo-Sastre A, Leverentz MK, Mills CG, Woodcock S, Baron M, Collu GM, Brennan K. Acar A, et al. Sci Rep. 2021 Apr 27;11(1):9096. doi: 10.1038/s41598-021-88618-5. Sci Rep. 2021. PMID: 33907274 Free PMC article. - Notch signaling pathway in cancer: from mechanistic insights to targeted therapies.
Shi Q, Xue C, Zeng Y, Yuan X, Chu Q, Jiang S, Wang J, Zhang Y, Zhu D, Li L. Shi Q, et al. Signal Transduct Target Ther. 2024 May 27;9(1):128. doi: 10.1038/s41392-024-01828-x. Signal Transduct Target Ther. 2024. PMID: 38797752 Free PMC article. Review. - A maternal dorsoventral prepattern revealed by an asymmetric distribution of ventralizing molecules before fertilization in Xenopus laevis.
Castro Colabianchi AM, González Pérez NG, Franchini LF, López SL. Castro Colabianchi AM, et al. Front Cell Dev Biol. 2024 Mar 20;12:1365705. doi: 10.3389/fcell.2024.1365705. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38572484 Free PMC article. - Repression of Wnt/β-catenin signaling by SOX9 and Mastermind-like transcriptional coactivator 2.
Sinha A, Fan VB, Ramakrishnan AB, Engelhardt N, Kennell J, Cadigan KM. Sinha A, et al. Sci Adv. 2021 Feb 17;7(8):eabe0849. doi: 10.1126/sciadv.abe0849. Print 2021 Feb. Sci Adv. 2021. PMID: 33597243 Free PMC article.
References
- Artavanis-Tsakonas S, Rand M. D, Lake R. J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Hartenstein A. Y, Rugendorff A, Tepass U, Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–1220. - PubMed
- Hayward P, Kalmar T, Martinez Arias A. Wnt/Notch signalling and information processing during development. Development. 2008;135:411–424. - PubMed
- Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–R138. - PubMed
- Ehebauer M, Hayward P, Arias A. M. Notch, a universal arbiter of cell fate decisions. Science. 2006;314:1414–1415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials