NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas - PubMed (original) (raw)
NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas
Ta-Jen Liu et al. Mol Cancer Ther. 2009 Aug.
Abstract
Aberrant genetic alternations in human gliomas, such as amplification of epidermal growth factor receptor, mutation and/or deletion of tumor suppressor gene PTEN, and mutations of PIK3CA, contribute to constitutive activation of the phosphatidylinositol 3-kinase (PI3K) pathway. We investigated the potential antitumor activity of NVP-BEZ235, which is a novel dual PI3K/mammalian target of rapamycin (mTOR) inhibitor in gliomas. The compound suppressed glioma cell proliferation with IC(50) values in the low nanomolar range by specifically inhibiting the activity of target proteins including Akt, S6K1, S6, and 4EBP1 in the PI3K/Akt/mTOR signaling pathway. NVP-BEZ235 treatment of glioma cell lines led to G(1) cell cycle arrest and induced autophagy. Furthermore, expression of the vascular endothelial growth factor (VEGF), which is an important angiogenic modulator in glioma cells, was significantly decreased, suggesting that NVP-BEZ235 may also exert an antiangiogenic effect. Preclinical testing of the therapeutic efficacy of NVP-BEZ235 showed that it significantly prolonged the survival of tumor-bearing animals without causing any obvious toxicity. Tumor extracts harvested from animals after treatment showed that the compound inhibited the activity of target proteins in the PI3K/Akt/mTOR cascade. Immunohistochemical analyses also showed a significant reduction in staining for VEGF von Willebrand factor (factor VIII) in NVP-BEZ235-treated tumor sections compared with controls, further confirming that NVP-BEZ235 has an antiangiogenic effect in vivo. We conclude from these findings that NVP-BEZ235 antagonizes PI3K and mTOR signaling and induces cell cycle arrest, down-regulation of VEGF, and autophagy. These results warrant further development of NVP-BEZ235 for clinical trials for human gliomas or other advanced cancers with altered PI3K/Akt/mTOR signaling.
Figures
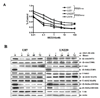
Figure 1. NVP-BEZ235 treatment inhibits glioma proliferation and attenuates PI3K/mTOR signaling pathway
(A) Glioma cells in 96-well plates were treated with increasing concentrations of NVP-BEZ235 for 72 h and subjected to an SRB assay, as described in Materials and Methods. Plot depicts the percentage growth of NVP-BEZ235-treated cells compared with the growth of the vehicle-treated control cells. Each culture was performed in triplicate. The results shown are the arithmetic mean ± the standard deviation from a single experiment. Similar results were obtained from three independent experiments. U251 and U87 cells are used as PTEN negative cells and LN18 and LN229 as PTEN positive cells. (B) To evaluate the target inactivation by NVP-BEZ235 in a time-dependent manner, glioma cells were treated with a fixed concentration of NVP-BEZ235 72 h. At each indicated time point, cell extracts were subjected to immunoblotting analysis.
Figure 2. NVP-BEZ235 interrupts growth factor-induced PI3K/mTOR signaling
Under basal conditions in serum-free medium (SFM), the PI3K signal as assessed by the phospho-Akt level was constitutively active in PTEN-negative U87 cells but not in wt-PTEN LN229 cells. Upon growth factor stimulation, levels of phospho-Akt in U87 cells remained unchanged, whereas a different pattern of phospho-Akt activation was noted in LN229 cells. Based on the extent of Akt activation, receptors for IGF-I seemed to be more abundant than receptors for EGF and PDGF in LN229 cells. The VEGF receptor appeared to be scarce in LN229 cells. The activity of Akt and S6K1, in both cell lines was significantly inhibited by NVP-BEZ235, suggesting its effectiveness on blocking the PI3K/mTOR signal pathway. Expression of the corresponding total target protein and actin was used as control.
Figure 3. NVP-BEZ235 causes cell cycle arrest and induces autophagy
(A) Cell cycle analysis was performed on cells treated or untreated with 50 nM NVP-BEZ235 for 48 h. Cell cycle distribution was labeled on the histogram. NVP-BEZ235 treatment resulted in G1 cell cycle arrest. (B) Following NVP-BEZ235 treatment, glioma cells were either stained with acridine orange for FACS analysis, to assess autophagy, or harvested for immunoblotting analysis, to assess LC3-II expression. Top panel shows the results of FACS analysis of acridine-positive cells. It appears that glioma cells are prone to NVP-BEZ235-induced autophagy. Bottom panel shows LC3-II expression as an indication of autophagy in the (−) untreated and (+) NVP-BEZ235-treated cells.
Figure 4. NVP-BEZ235 treatment prolongs survival of animals with intracranial xenografts
(A) Top panel: The Kaplan-Meier survival curve for the U87 xenograft experiment, with a p value of 0.026. The p values in the plot determined by the log-rank test are for the comparison of the overall survival of the vehicle-treated mice with that of the NVP-BEZ235-treated mice. (B) Weight measurements of experimental animals ar regular intervals. (C) Tumor volumes of intracranial tumors were measured at the time of sacrifice. The mean tumor volume was reduced in treated animal with NVP-BEZ235. (D) Immunoblotting analyses to assess LC3-II expression after 2-week and 4-week tumor cell extracts following NVP-BEZ235 treatment. The increase in ratio of LC3-II/LC3-I is an indication of autophagy in NVP-BEZ235 treated tumors in comparison to vehicle treated animals. (E) Immunoblotting analyses of both the expression and activation of Akt and S6K1 in 2-week and 4-week tumors following NVP-BEZ235 treatment. Immunoblotting analyses demonstrated that NVP-BEZ235 inhibited the activity of S6K1 in both sets of tumors, as assessed by the level of corresponding phosphorylated protein.
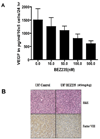
Figure 5. NVP-BEZ235 attenuates VEGF secretion and inhibits factor VIII expression VEGF expression in-vivo
(A) NVP-BEZ235 attenuated VEGF secretion by at least 40% in U87 cells. The VEGF level in the serum-free medium was measured in triplicate and the experiment repeated at least twice to confirm results. The amount of secreted VEGF was rendered in pg/ml/105 cells/24 h, as described previously (26). (B) Detection of factor VIII. Staining of factor VIII was used to measure the effect of NVP-BEZ235 on tumor angiogenesis. A drastic reduction in factor VIII–positive staining was noted in the NVP-BEZ235-treated (45mg/Kg) tumor section as compared with staining results in control cells.
Similar articles
- Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 has a therapeutic potential and sensitizes cisplatin in nasopharyngeal carcinoma.
Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin J, Feng GK, Zhu XF. Yang F, et al. PLoS One. 2013;8(3):e59879. doi: 10.1371/journal.pone.0059879. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533654 Free PMC article. - NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells.
Yu Z, Xie G, Zhou G, Cheng Y, Zhang G, Yao G, Chen Y, Li Y, Zhao G. Yu Z, et al. Cancer Lett. 2015 Oct 10;367(1):58-68. doi: 10.1016/j.canlet.2015.07.007. Epub 2015 Jul 15. Cancer Lett. 2015. PMID: 26188279 - Genotype-dependent efficacy of a dual PI3K/mTOR inhibitor, NVP-BEZ235, and an mTOR inhibitor, RAD001, in endometrial carcinomas.
Shoji K, Oda K, Kashiyama T, Ikeda Y, Nakagawa S, Sone K, Miyamoto Y, Hiraike H, Tanikawa M, Miyasaka A, Koso T, Matsumoto Y, Wada-Hiraike O, Kawana K, Kuramoto H, McCormick F, Aburatani H, Yano T, Kozuma S, Taketani Y. Shoji K, et al. PLoS One. 2012;7(5):e37431. doi: 10.1371/journal.pone.0037431. Epub 2012 May 25. PLoS One. 2012. PMID: 22662154 Free PMC article. - Xanthatin suppresses proliferation and tumorigenicity of glioma cells through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway.
Chen H, Zhu T, Huang X, Xu W, Di Z, Ma Y, Xue M, Bi S, Shen Y, Yu Y, Shen Y, Feng L. Chen H, et al. Pharmacol Res Perspect. 2023 Feb;11(1):e01041. doi: 10.1002/prp2.1041. Pharmacol Res Perspect. 2023. PMID: 36572650 Free PMC article. Review. - Role of dual PI3/Akt and mTOR inhibition in Waldenstrom's Macroglobulinemia.
Sacco A, Roccaro A, Ghobrial IM. Sacco A, et al. Oncotarget. 2010 Nov;1(7):578-582. doi: 10.18632/oncotarget.192. Oncotarget. 2010. PMID: 21317453 Free PMC article. Review.
Cited by
- Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 has a therapeutic potential and sensitizes cisplatin in nasopharyngeal carcinoma.
Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin J, Feng GK, Zhu XF. Yang F, et al. PLoS One. 2013;8(3):e59879. doi: 10.1371/journal.pone.0059879. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533654 Free PMC article. - Autophagy inhibition enhances colorectal cancer apoptosis induced by dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235.
Yang X, Niu B, Wang L, Chen M, Kang X, Wang L, Ji Y, Zhong J. Yang X, et al. Oncol Lett. 2016 Jul;12(1):102-106. doi: 10.3892/ol.2016.4590. Epub 2016 May 17. Oncol Lett. 2016. PMID: 27347108 Free PMC article. - PI3K and Akt as molecular targets for cancer therapy: current clinical outcomes.
Pal I, Mandal M. Pal I, et al. Acta Pharmacol Sin. 2012 Dec;33(12):1441-58. doi: 10.1038/aps.2012.72. Epub 2012 Sep 17. Acta Pharmacol Sin. 2012. PMID: 22983389 Free PMC article. Review. - NVP-BEZ235 overcomes gefitinib-acquired resistance by down-regulating PI3K/AKT/mTOR phosphorylation.
Sun Z, Li Q, Zhang S, Chen J, Huang L, Ren J, Chang Y, Liang Y, Wu G. Sun Z, et al. Onco Targets Ther. 2015 Jan 29;8:269-77. doi: 10.2147/OTT.S62128. eCollection 2015. Onco Targets Ther. 2015. PMID: 25674002 Free PMC article. - NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro.
Wang WJ, Long LM, Yang N, Zhang QQ, Ji WJ, Zhao JH, Qin ZH, Wang Z, Chen G, Liang ZQ. Wang WJ, et al. Acta Pharmacol Sin. 2013 May;34(5):681-90. doi: 10.1038/aps.2013.22. Epub 2013 Apr 22. Acta Pharmacol Sin. 2013. PMID: 23603977 Free PMC article.
References
- Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. - PubMed
- Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. - PubMed
- Gallia GL, Rand V, Siu IM, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709–714. - PubMed
- Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3’-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585–3592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- R01 CA56041/CA/NCI NIH HHS/United States
- P50 CA127001-01A1/CA/NCI NIH HHS/United States
- P50 CA127001/CA/NCI NIH HHS/United States
- CA123304/CA/NCI NIH HHS/United States
- P50 CA127001-01A10002/CA/NCI NIH HHS/United States
- R01 CA123304/CA/NCI NIH HHS/United States
- P50CA127001/CA/NCI NIH HHS/United States
- R01 CA056041-15/CA/NCI NIH HHS/United States
- R01 CA056041/CA/NCI NIH HHS/United States
- R01 CA123304-03/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous