Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival - PubMed (original) (raw)
doi: 10.1186/1756-6606-2-27.
Kazuyuki Kiyosue, Tomoko Hara, Shunsuke Hazama, Shingo Suzuki, Koichi Uegaki, Guhan Nagappan, Eugene Zaitsev, Takatsugu Hirokawa, Yoshiro Tatsu, Akihiko Ogura, Bai Lu, Masami Kojima
Affiliations
- PMID: 19674479
- PMCID: PMC2743674
- DOI: 10.1186/1756-6606-2-27
Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival
Hisatsugu Koshimizu et al. Mol Brain. 2009.
Abstract
Background: Proneurotrophins and mature neurotrophins elicit opposite effects via the p75 neurotrophin receptor (p75(NTR)) and Trk tyrosine kinase receptors, respectively; however the molecular roles of proneurotrophins in the CNS are not fully understood.
Results: Based on two rare single nucleotide polymorphisms (SNPs) of the human brain-derived neurotrophic factor (BDNF) gene, we generated R125M-, R127L- and R125M/R127L-BDNF, which have amino acid substitution(s) near the cleavage site between the pro- and mature-domain of BDNF. Western blot analyses demonstrated that these BDNF variants are poorly cleaved and result in the predominant secretion of proBDNF. Using these cleavage-resistant proBDNF (CR-proBDNF) variants, the molecular and cellular roles of proBDNF on the CNS neurons were examined. First, CR-proBDNF showed normal intracellular distribution and secretion in cultured hippocampal neurons, suggesting that inhibition of proBDNF cleavage does not affect intracellular transportation and secretion of BDNF. Second, we purified recombinant CR-proBDNF and tested its biological effects using cultured CNS neurons. Treatment with CR-proBDNF elicited apoptosis of cultured cerebellar granule neurons (CGNs), while treatment with mature BDNF (matBDNF) promoted cell survival. Third, we examined the effects of CR-proBDNF on neuronal morphology using more than 2-week cultures of basal forebrain cholinergic neurons (BFCNs) and hippocampal neurons. Interestingly, in marked contrast to the action of matBDNF, which increased the number of cholinergic fibers and hippocampal dendritic spines, CR-proBDNF dramatically reduced the number of cholinergic fibers and hippocampal dendritic spines, without affecting the survival of these neurons.
Conclusion: These results suggest that proBDNF has distinct functions in different populations of CNS neurons and might be responsible for specific physiological cellular processes in the brain.
Figures
Figure 1
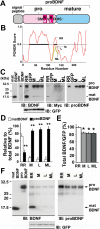
BDNF polymorphic substitutions inhibit the conversion of proBDNF to matBDNF and lead to predominant secretion of proBDNF in CNS neurons. (A) Schematic of human BDNF protein. Amino acid substitutions near the cleavage site in rare SNP variants are represented by single-letter symbols. The amino acid substitutions caused by the SNPs are depicted by white symbols. (B) Bioinformatic prediction of changes in the secondary structure of proBDNF SNPs variants using PONDR. M, L and ML depict the amino acid substitution of R125M, R127L and R125M/R127L, respectively. The disordered regions (PONDR score > 0.5) of wild-type BDNF (aa 25–103 and 120–140) is highlighted by bold lines. (C) Inhibition of the intracellular cleavage of SNP variants in cultured hippocampal neurons. The cultures were infected with Sindbis viruses expressing wild-type (RR), R125M (M), R127L (L), and R125M/R127L (ML) constructs for 12 h and 3 days later processed for Western blot analysis using anti-BDNF (IB: BDNF) and anti-Myc antibodies (IB: Myc). Doublet bands of 29- and 32-kDa were detected with the monoclonal anti-proBDNF antibody (IB: proBDNF), whereas an additional band of 14-kDa (matBDNF) was detected using an antibody against the mature domain (IB: BDNF). The levels of bicistronically expressed GFP, which correlated with the viral infection levels, were similar in all cultures. Note that proBDNF bands were predominant in the lysates of cells expressing the M, L, and ML constructs, while matBDNF expression was residual. In C and F, 1 ng recombinant matBDNF and proBDNF (R125M/R127L-BDNF) were used as positive controls. (D) The ratio of proBDNF to matBDNF was quantified by densitometric analysis on BDNF, and proBDNF bands. **P < 0.01 (Student's _t_-test) when compared with matBDNF (matBDNFRR + proBDNFRR = 100% as control). n = 3 independent experiments. (E) The entire amount of BDNF was quantified by densitometric analysis on matBDNF, proBDNF, and GFP bands. *P < 0.05 (_t_-test) when compared with RR (100% as control). n = 3 independent experiments. (F) Secretion of poorly cleaved proBDNF. Cultured cerebral cortical neurons were incubated with Sindbis viruses for 12 h and maintained in serum-containing medium for 3 days. Supernatants were collected and immunoprecipitated using anti-Myc antibody-conjugated agarose beads, followed by Western blot analysis using rabbit antibody against the mature domain (IB: BDNF) and mouse antibody recognizing the prodomain (IB: proBDNF). Cell lysates were blotted with anti-GFP antibody to normalize viral infection (IB: GFP).
Figure 2
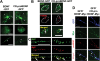
Intracellular proteolytic cleavage of proBDNF is not a crucial step for intracellular transportation and secretion of BDNF in hippocampal neurons. (A) Distribution of GFP-tagged wild-type BDNF (BDNF-GFP) and cleavage-resistant (CR)-proBDNF (R125M/R127L-BDNF-GFP) in the cell body and processes. Cultured neurons were subjected to Sindbis viruses expressing the indicated constructs for 3 h. Three days later, the neurons were fixed for imaging of GFP fluorescence using confocal microscope. Representative low-magnification (upper) and high-magnification images of the cell body (middle) and neuronal processes (bottom) are shown. (B-C) The infected cells were immunostained with the indicated antibodies for confocal imaging. Note that CR-proBDNF-GFP greatly co-localized with TGN38 (B) and SgII (C), similarly to wild-type BDNF-GFP (arrows). Scale bar in all images, 10 μm. (D) Expression of wild-type proBDNF-Myc or CR-proBDNF-Myc. Hippocampal neurons were introduced with constructs encoding GFP and wild-type BDNF-Myc or CR-proBDNF-Myc using Sindbis virus expression system. Three-days after a brief (3 h) infection, cells were double-stained using anti-Myc and anti-proBDNF antibodies. BDNF-Myc signals largely co-localized with proBDNF signals (arrows) indicating that proBDNF is the predominant intracellular isoform. Scale bar, 5 μm.
Figure 3
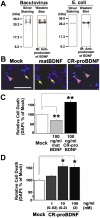
Recombinant CR-proBDNF induces apoptosis of cerebellar granule neurons cultured in low K+-containing medium. (A) Preparation of recombinant CR-proBDNF (R125M/R127L-BDNF: proBDNFML) using the Baculovirus or E. coli expression systems. Protein products were analyzed by silver staining (left) and Western blotting using rabbit antibody against BDNF prodomain. (B-C) Opposite actions of CR-proBDNF and matBDNF revealed by apoptosis assay. After 4 days of culture in HK medium, CGNs were treated with _E. coli_-derived CR-proBDNF or matBDNF in LK medium. Cell death was assessed using DAPI staining 48 h after treatment with the indicated drug in LK medium. (B) Arrows and arrowheads indicate representative living and dead cells, respectively. Scale bar, 50 μm. (C) For quantification of cell death, 300–400 cells were counted in four independent fields per coverslip. (D) Dose-dependent test of pro-apoptotic activity of CR-proBDNF. Cell death was assessed using DAPI staining 48 h after treatment with CR-proBDNF in LK medium. DAPI data were expressed as the percentage of mock cultures. In the cell death assay (C-D), n = 4 independent culture dishes. ANOVA followed by post-hoc analysis, *P < 0.05; **P < 0.01. Results were replicated in at least three independent experiments.
Figure 4
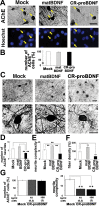
Neurite growth of basal forebrain cholinergic neurons is inhibited by CR-proBDNF, but elicited by matBDNF. BFCNs were cultured in serum-containig (A-E) or serum-free (F) medium for 2 weeks and treated with 100 ng/ml CR-proBDNF or matBDNF in the same medium condition for 2 days. Histochemistry and quantitation of AChE-positve neurites were done as described in Methods. (A) BFCNs were double-stained using AChE histochemistry and Hoechst 33258 (arrows). (B) The survival rate of BFCNs (%) = 100 × [living cells]/([living cells] + [dead cells]). Data were normalized to mock cultures (100% as control). n = 159 (Mock), 151 (matBDNF), and 149 (CR-proBDNF) from three independent coverslips. Results were replicated in three independent experiments. (C) Low- and high-magnification images of AChE-stained BFCNs. (D) The number of neurites extending outwards from the cell body shown in C. n = 38 (Mock), 37 (matBDNF), and 40 (CR-proBDNF) cells from three independent coverslips. (E) Neurite complexity as revealed by Sholl analysis. n = 30 (Mock), 30 (matBDNF), and 30 (CR-proBDNF) cells from three independent coverslips. In multi-bar graphs, ANOVA followed by post-hoc analysis was used. **P < 0.01. Results were replicated in at least three independent experiments. (F) The opposing effects of matBDNF and CR-proBDNF on neurite fiber density were confirmed by a distinct quantitative method. The maximal threshold of AChE-positive cholinergic fiber intensity was defined as 70% above the background. The total intensity of the fibers was determined in an optical field and was divided by the number of AChE-positive neurons in the same field. Data were collected from four independent fields in a single chamber. _t_-test, **P < 0.01, compared to Mock (100% as control); n = 6 independent culture dishes. Scale bar, 5 μm (A and C). (G) Effect of CR-proBDNF on neurite density of BFCNs in serum-free condition and a dose-dependency test of CR-proBDNF. Cell survival (left) and the neurite number of BFCNs (right) were determined. Note that proBDNF negatively regulates the neurite density of BFCNs in serum-free conditions and at subnanomoler concentration. n = 3 independent coverslips. _t_-test, **P < 0.01, significantly different from Mock.
Figure 5
proBDNF reduces dendritic spine density in hippocampal neurons. Cultured neurons were maintained for 3–4 weeks and treated with the indicated reagents (50 ng/ml) for 2 (A, B, and D) or 3 days (C). In A and B, neurons were cultured in serum-containing medium. (A) CR-proBDNF reduced the density of DiI-labeled spines. Representative images of DiI-labeled neurons treated with the indicated reagents for 2 days (left). Summary of spine density (middle) and length (right). Data were collected from 52 (Mock), 59 (matBDNF), and 38 (CR-proBDNF) independent cells (non-parametric test, *P < 0.05, compared to Mock). Note that matBDNF increased spine density, while proBDNF reduced spine density (arrows and arrowheads). (B) proBDNF promoted the shrinkage of phalloidin-labeled spines and p75NTR was involved in the proBDNF action. Application of a functionally blocking antibody against p75NTR, AB1554 was performed and fixed neurons were stained with an anti-MAP2 antibody and FITC-labeled phalloidin. Representative images of the double-stained neurons (left) and a quantitative analysis of phalloidin-labeled spiny structures (right) are shown, n = 30–40 independent cells from three independent coverslips. _t_-test, *P < 0.05, **P < 0.01, compared to Mock (100% as control). (C) CR-proBDNF decreases spine density in low-density hippocampal neurons cultured in serum-free medium. The cultures were incubated with CR-proBDNF in serum-free medium for 2 days and double-stained with an anti-MAP2 antibody and FITC-labeled phalloidin. n = 24–32 cells from three independent coverslips. _t_-test, *P < 0.05, **P < 0.01, compared to Mock. (D) CR-proBDNF decreased spine density in hippocampal slices. Representative images of DiI-labeled neurons at low and high magnification are shown (left). A decrease in spine density was observed in CR-proBDNF-treated slices (arrow heads). Quantification of spine density (right). Data were collected from 7 (Mock) and 6 (CR-proBDNF) independent slices. Note that CR-proBDNF-treated slices showed a decrease in spine density when compared with matBDNF. _t_-test, *P < 0.05. Scale bar, 10 μm (A, B, and D).
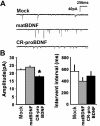
Figure 6
CR-proBDNF attenuates amplitude of functional synaptic transmission in cultured hippocampal neurons. Cells were cultured as described in Fig. 5A and treated with the indicated reagents for 3 days. A patch-clamp technique in the whole-cell configuration was used to record mEPSCs from pyramidal-shaped neurons and all data were statistically evaluated. (A) Representative mEPSCs. (B) Amplitude (left) and frequency (right) of mEPSCs. Note that proBDNF significantly decreased the amplitude, but not the frequency, of mEPSCs. Data were collected from 27–31 independent cells (non-parametric test, *P < 0.05, significantly different from Mock).
Similar articles
- Distinct signaling pathways of precursor BDNF and mature BDNF in cultured cerebellar granule neurons.
Koshimizu H, Hazama S, Hara T, Ogura A, Kojima M. Koshimizu H, et al. Neurosci Lett. 2010 Apr 12;473(3):229-32. doi: 10.1016/j.neulet.2010.02.055. Epub 2010 Feb 26. Neurosci Lett. 2010. PMID: 20219632 - proBDNF is modified by advanced glycation end products in Alzheimer's disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing.
Fleitas C, Piñol-Ripoll G, Marfull P, Rocandio D, Ferrer I, Rampon C, Egea J, Espinet C. Fleitas C, et al. Mol Brain. 2018 Nov 14;11(1):68. doi: 10.1186/s13041-018-0411-6. Mol Brain. 2018. PMID: 30428894 Free PMC article. - proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus.
Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL. Yang J, et al. Cell Rep. 2014 May 8;7(3):796-806. doi: 10.1016/j.celrep.2014.03.040. Epub 2014 Apr 17. Cell Rep. 2014. PMID: 24746813 Free PMC article. - [Diversity of proBDNF and mBDNF functions in the central nervous system].
Borodinova AA, Salozhin SV. Borodinova AA, et al. Zh Vyssh Nerv Deiat Im I P Pavlova. 2016 Jan-Feb;66(1):3-23. Zh Vyssh Nerv Deiat Im I P Pavlova. 2016. PMID: 27263272 Review. Russian. - Peptides other than the neurotrophins that can be cleaved from proneurotrophins: a neglected story.
Dicou E. Dicou E. Arch Physiol Biochem. 2007 Oct-Dec;113(4-5):228-33. doi: 10.1080/13813450701531250. Arch Physiol Biochem. 2007. PMID: 17917853 Review.
Cited by
- Matrix Metalloproteinase-9 Regulates Neuronal Circuit Development and Excitability.
Murase S, Lantz CL, Kim E, Gupta N, Higgins R, Stopfer M, Hoffman DA, Quinlan EM. Murase S, et al. Mol Neurobiol. 2016 Jul;53(5):3477-3493. doi: 10.1007/s12035-015-9295-y. Epub 2015 Jun 21. Mol Neurobiol. 2016. PMID: 26093382 Free PMC article. - Frontotemporal Transcranial Direct Current Stimulation Decreases Serum Mature Brain-Derived Neurotrophic Factor in Schizophrenia.
Adam O, Psomiades M, Rey R, Mandairon N, Suaud-Chagny MF, Mondino M, Brunelin J. Adam O, et al. Brain Sci. 2021 May 19;11(5):662. doi: 10.3390/brainsci11050662. Brain Sci. 2021. PMID: 34069556 Free PMC article. - Phosphodiesterase 4 inhibition after retrieval switches the memory fate favoring extinction instead of reconsolidation.
Machado Batista Sohn J, Cardoso NC, Raymundi AM, Prickaerts J, Stern CAJ. Machado Batista Sohn J, et al. Sci Rep. 2023 Nov 21;13(1):20384. doi: 10.1038/s41598-023-47717-1. Sci Rep. 2023. PMID: 37990053 Free PMC article. - Sex-dependent effect of the BDNF Val66Met polymorphism on executive functioning and processing speed in older adults: evidence from the health ABC study.
Barha CK, Liu-Ambrose T, Best JR, Yaffe K, Rosano C; Health, Aging and Body Composition Study. Barha CK, et al. Neurobiol Aging. 2019 Feb;74:161-170. doi: 10.1016/j.neurobiolaging.2018.10.021. Epub 2018 Oct 23. Neurobiol Aging. 2019. PMID: 30448615 Free PMC article. - Retrograde Axonal Transport of Neurotrophins in Basal Forebrain Cholinergic Neurons.
Shekari A, Fahnestock M. Shekari A, et al. Methods Mol Biol. 2022;2431:249-270. doi: 10.1007/978-1-0716-1990-2_13. Methods Mol Biol. 2022. PMID: 35412281
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials