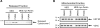Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release - PubMed (original) (raw)
. 2009 Nov 15;18(22):4317-28.
doi: 10.1093/hmg/ddp384. Epub 2009 Aug 13.
Affiliations
- PMID: 19679562
- PMCID: PMC2766292
- DOI: 10.1093/hmg/ddp384
Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release
Alison K Berger et al. Hum Mol Genet. 2009.
Abstract
Autosomal-recessive mutations in the Parkin gene are the second most common cause of familial Parkinson's disease (PD). Parkin deficiency leads to the premature demise of the catecholaminergic neurons of the ventral midbrain in familial PD. Thus, a better understanding of parkin function may elucidate molecular aspects of their selective vulnerability in idiopathic PD. Numerous lines of evidence suggest a mitochondrial function for parkin and a protective effect of ectopic parkin expression. Since mitochondria play a critical role in cell survival/cell death through regulated cytochrome c release and control of apoptosis, we sought direct evidence of parkin function in this pathway. Mitochondria were isolated from cells expressing either excess levels of human parkin or shRNA directed against endogenous parkin and then treated with peptides corresponding to the active Bcl-2 homology 3 (BH3) domains of pro-apoptotic proteins and the threshold for cytochrome c release was analyzed. Data obtained from both rodent and human neuroblastoma cell lines showed that the expression levels of parkin were inversely correlated with cytochrome c release. Parkin was found associated with isolated mitochondria, but its binding per se was not sufficient to inhibit cytochrome c release. In addition, pathogenic parkin mutants failed to influence cytochrome c release. Furthermore, PINK1 expression had no effect on cytochrome c release, suggesting a divergent function for this autosomal recessive PD-linked gene. In summary, these data demonstrate a specific autonomous effect of parkin on mitochondrial mechanisms governing cytochrome c release and apoptosis, which may be relevant to the selective vulnerability of certain neuronal populations in PD.
Figures
Figure 1.
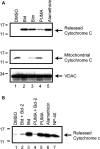
BH3 peptides induce cytochrome c release from isolated mitochondria. (A) Mitochondria were isolated from MES cells and incubated with DMSO, 10 µ
m
of the purified BH3 domain peptides of Bid, Bim, PUMA or alamethicin (40 µg/ml). Reactions were then centrifuged at 25 000_g_ for 10 min, to obtain the released cytochrome c supernatant and mitochondrial pellet. Released and retained cytochrome c was analyzed by western blot. Equal loading of mitochondrial proteins was confirmed by western blotting for the mitochondrial membrane protein, VDAC. (B) Mitochondria were pre-incubated for 5 min with purified, full-length Bcl-2 protein (10 µ
m
), prior to the addition of 10 µ
m
Bid or PUMA BH3 peptide. Released cytochrome c was measured by western blot. Images are representative of at least three independent experiments.
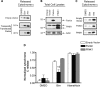
Figure 2.
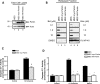
The anti-apoptotic effects of parkin are intrinsic to the mitochondria and can be observed in a cell-free system. (A) Human parkin was stably expressed in two independent monoclonal MES cell lines. Whole cell lysates were probed for parkin and actin expression by western blot. (B) Mitochondria were isolated from MES, MES myc-Parkin and MES HA parkin cells, protein normalized and incubated with DMSO, or 10 µ
m
of the purified BH3 domain peptides of Bid and Bim at various concentrations. NP-40 (1%) was added to aliquots of each mitochondrial preparation to measure total cytochrome c from each sample. Released cytochrome c was analyzed by western blot. (C) Caspase 3/7 activity was measured from MES and MES myc-Parkin cells 18 h after the treatment with vehicle or 1 µ
m
C2 ceramide (mean ± SEM, n = 20). Data were pooled across three independent experiments, # denotes statistically significant from control (P < 0.00001). (D) MES, MES myc-Parkin and MES HA-Parkin cells were treated with vehicle, 1 µ
m
C2 ceramide or 300 n
m
staurosporine for 6 h and caspase 3/7 activity was measured (mean ± SEM, n = 4), *denotes statistically significant from control (P < 0.001).
Figure 3.
Parkin prevents PUMA peptide-induced cytochrome c release from mitochondria. (A) Mitochondria from the MES and MES myc-Parkin cell lines were incubated with either DMSO or the purified BH3 domain of PUMA (10 µ
m
). Released cytochrome c was detected by western blot. (B) Equal loading of mitochondrial proteins was confirmed by western blotting the mitochondrial retained proteins for HSP 60 and VDAC. Images are representative of at least three independent experiments, each performed in duplicate as shown.
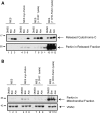
Figure 4.
Pathogenic parkin mutations do not inhibit mitochondrial cytochrome c release. (A) Parental MES cells (MES) and lines stably expressing wild-type parkin (WT Parkin) or parkin mutants R275W and W453X were lysed in NP-40 and immunoblotted for parkin expression and actin levels as a loading control. (B) WT parkin and mutant-parkin expressing cell lines were incubated with either DMSO, 10 µ
m
of the purified BH3 domain peptides of Bid or Bim, or alamethicin (Alm, 40 µg/ml). Released cytochrome c was quantified by ELISA with the data normalized to maximal cytochrome c release as determined by alamethicin treatment from each mitochondrial preparation. * Denotes statistically significant from control (P < 0.05, n = 3). (C) Western blot of a representative experiment reveals the failure of mutant parkin to influence mitochondrial cytochrome c release whereas wild-type parkin dramatically reduces BH3 peptide induced cytochrome c release.
Figure 5.
The association of parkin with mitochondria is not sufficient for inhibition of evoked cytochrome c release. Mitochondria from the MES and MES myc-Parkin cell lines were incubated with either DMSO, the purified BH3 domain of Bim (10 µ
m
), or alamethicin (Alm, 40 µg/ml) with normal Experimental Buffer. In addition, mitochondria isolated from parental MES cells were incubated with soluble parkin-free extract from CHO− cells, or parkin-enriched extract from CHO-Parkin cells. Reactions were then centrifuged at 25 000_g_ for 10 min, to obtain (A) the released cytochrome c supernatant which was probed for released cytochrome c and the presence of parkin and (B) the mitochondrial pellet which was probed for VDAC as a loading control, and for mitochondria-associated parkin. * Denotes a non-specific signal in a non-loaded lane coming from the adjacent lane. This image is a representative of at least three independent experiments.
Figure 6.
Endogenous parkin regulates the apoptotic release of cytochrome c from mitochondria. (A) The ectopic expression of parkin in SH-SY5Y cells substantially attenuates the BH3 peptide induce cytochrome c release from isolated mitochondria, with a concomitant decrease in the retained cytochrome c (B), as determined by western blot. Equal loading of mitochondrial proteins was confirmed by western blot analysis of VDAC. (C) Whole cell lysates from naïve SH-SY5Y cells and those stably transduced with a lentivirus encoding an shRNA directed against the expression of endogenous parkin were probed by western blot for both parkin and actin levels. (D) Mitochondria from SH-SY5Y cell lines with and without shRNA against parkin expression were incubated with DMSO vehicle, 1 µ
m
of the purified BH3 domain peptides of Bid, Bim and PUMA, alamethicin (40 µg/ml), or 1% NP-40. Released and retained cytochrome c, and mitochondrial VDAC levels were analyzed by western blot. (D) is representative of at least three independent experiments.
Figure 7.
PINK1 does not prevent BH3 peptide-induced cytochrome c release (A) A representative western blot analysis of released cytochrome c release in a BH3 peptide assay following transient transfection to express parkin at 24 and 48 h. (B) Western blot analysis of whole cell lysates from MES cells lines transiently transfected with empty vector, wild-type parkin or PINK1. (C) Mitochondria were isolated 48 h after transient transfection with an empty vector control, parkin, or PINK1 and subject to a BH3 peptide assay. Western blot analysis of the Bim (10 µ
m
) and alamethicin (Alm, 40 µg/ml) induced cytochrome c release demonstrates the effects of transiently expressed parkin and PINK1 on cytochrome c release. (D) Data from three independent experiments were analyzed by densitometry and normalized to the maximal cytochrome c release from each mitochondrial preparation (as determined by alamethicin treatment). *Denotes statistically significant from control (P < 0.05, n = 3) and † is significant from PINK1 (P < 0.05, n = 3).
Similar articles
- Parkin promotes degradation of the mitochondrial pro-apoptotic ARTS protein.
Kemeny S, Dery D, Loboda Y, Rovner M, Lev T, Zuri D, Finberg JP, Larisch S. Kemeny S, et al. PLoS One. 2012;7(7):e38837. doi: 10.1371/journal.pone.0038837. Epub 2012 Jul 9. PLoS One. 2012. PMID: 22792159 Free PMC article. - Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin.
Charan RA, Johnson BN, Zaganelli S, Nardozzi JD, LaVoie MJ. Charan RA, et al. Cell Death Dis. 2014 Jul 3;5(7):e1313. doi: 10.1038/cddis.2014.278. Cell Death Dis. 2014. PMID: 24991765 Free PMC article. - Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson's disease.
Koentjoro B, Park JS, Sue CM. Koentjoro B, et al. Sci Rep. 2017 Mar 10;7:44373. doi: 10.1038/srep44373. Sci Rep. 2017. PMID: 28281653 Free PMC article. - Parkin and PINK1 functions in oxidative stress and neurodegeneration.
Barodia SK, Creed RB, Goldberg MS. Barodia SK, et al. Brain Res Bull. 2017 Jul;133:51-59. doi: 10.1016/j.brainresbull.2016.12.004. Epub 2016 Dec 23. Brain Res Bull. 2017. PMID: 28017782 Free PMC article. Review. - Evidence for a common biological pathway linking three Parkinson's disease-causing genes: parkin, PINK1 and DJ-1.
van der Merwe C, Jalali Sefid Dashti Z, Christoffels A, Loos B, Bardien S. van der Merwe C, et al. Eur J Neurosci. 2015 May;41(9):1113-25. doi: 10.1111/ejn.12872. Epub 2015 Mar 11. Eur J Neurosci. 2015. PMID: 25761903 Review.
Cited by
- Parkin-mediated ubiquitination contributes to the constitutive turnover of mitochondrial fission factor (Mff).
Lee L, Seager R, Nakamura Y, Wilkinson KA, Henley JM. Lee L, et al. PLoS One. 2019 May 21;14(5):e0213116. doi: 10.1371/journal.pone.0213116. eCollection 2019. PLoS One. 2019. PMID: 31112535 Free PMC article. - Gene expression profiling specifies chemokine, mitochondrial and lipid metabolism signatures in leprosy.
Guerreiro LT, Robottom-Ferreira AB, Ribeiro-Alves M, Toledo-Pinto TG, Rosa Brito T, Rosa PS, Sandoval FG, Jardim MR, Antunes SG, Shannon EJ, Sarno EN, Pessolani MC, Williams DL, Moraes MO. Guerreiro LT, et al. PLoS One. 2013 Jun 14;8(6):e64748. doi: 10.1371/journal.pone.0064748. Print 2013. PLoS One. 2013. PMID: 23798993 Free PMC article. - Age-associated insolubility of parkin in human midbrain is linked to redox balance and sequestration of reactive dopamine metabolites.
Tokarew JM, El-Kodsi DN, Lengacher NA, Fehr TK, Nguyen AP, Shutinoski B, O'Nuallain B, Jin M, Khan JM, Ng ACH, Li J, Jiang Q, Zhang M, Wang L, Sengupta R, Barber KR, Tran A, Im DS, Callaghan S, Park DS, Zandee S, Dong X, Scherzer CR, Prat A, Tsai EC, Takanashi M, Hattori N, Chan JA, Zecca L, West AB, Holmgren A, Puente L, Shaw GS, Toth G, Woulfe JM, Taylor P, Tomlinson JJ, Schlossmacher MG. Tokarew JM, et al. Acta Neuropathol. 2021 May;141(5):725-754. doi: 10.1007/s00401-021-02285-4. Epub 2021 Mar 10. Acta Neuropathol. 2021. PMID: 33694021 Free PMC article. - Parkin inhibits BAK and BAX apoptotic function by distinct mechanisms during mitophagy.
Bernardini JP, Brouwer JM, Tan IK, Sandow JJ, Huang S, Stafford CA, Bankovacki A, Riffkin CD, Wardak AZ, Czabotar PE, Lazarou M, Dewson G. Bernardini JP, et al. EMBO J. 2019 Jan 15;38(2):e99916. doi: 10.15252/embj.201899916. Epub 2018 Dec 20. EMBO J. 2019. PMID: 30573668 Free PMC article. - The centrality of mitochondria in the pathogenesis and treatment of Parkinson's disease.
Camilleri A, Vassallo N. Camilleri A, et al. CNS Neurosci Ther. 2014 Jul;20(7):591-602. doi: 10.1111/cns.12264. Epub 2014 Apr 7. CNS Neurosci Ther. 2014. PMID: 24703487 Free PMC article. Review.
References
- Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
- Nussbaum R.L., Ellis C.E. Alzheimer's disease and Parkinson's disease. N. Engl. J. Med. 2003;348:1356–1364. - PubMed
- Leroy E., Anastasopoulos D., Konitsiotis S., Lavedan C., Polymeropoulos M.H. Deletions in the Parkin gene and genetic heterogeneity in a Greek family with early onset Parkinson's disease. Hum. Genet. 1998;103:424–427. - PubMed
- Lucking C.B., Abbas N., Durr A., Bonifati V., Bonnet A.M., de Broucker T., De Michele G., Wood N.W., Agid Y., Brice A. Homozygous deletions in parkin gene in European and North African families with autosomal recessive juvenile parkinsonism. The European consortium on genetic susceptibility in Parkinson's disease and the French Parkinson's disease genetics study group. Lancet. 1998;352:1355–1356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous