Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat - PubMed (original) (raw)
Comparative Study
. 2009 Nov;37(11):2163-70.
doi: 10.1124/dmd.109.028506. Epub 2009 Aug 13.
Affiliations
- PMID: 19679675
- PMCID: PMC2774984
- DOI: 10.1124/dmd.109.028506
Comparative Study
Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat
Michael H Baumann et al. Drug Metab Dispos. 2009 Nov.
Abstract
Based on animal data, there is speculation that (+ or -)-3,4-methylenedioxymethamphetamine (MDMA) is neurotoxic to humans. Extrapolation of MDMA findings from animals to humans requires assessment of pharmacokinetics in various species, and low-dose administration data from rats are lacking. In this study, we examine MDMA pharmacokinetics in rats given low (2 mg/kg) and high (10 mg/kg) doses of racemic MDMA via intraperitoneal, subcutaneous, and oral routes. Repeated blood specimens were collected from venous catheters, and plasma was assayed for MDMA and its metabolites, 4-hydroxy-3-methoxymethamphetamine (HMMA) and 3,4-methylenedioxyamphetamine (MDA), by gas chromatography-mass spectrometry. After 2 mg/kg, maximum MDMA concentrations (C(max)) were approximately 200 ng/ml for intraperitoneal and subcutaneous routes, but less for the oral route. MDMA plasma half-lives were <1 h for low-dose groups, whereas HMMA and MDA half-lives were >2 h. After 10 mg/kg, MDMA areas under the curve (AUCs) were 21-fold (intraperitoneal), 10-fold (subcutaneous), and 36-fold (oral) greater than those at 2 mg/kg. In contrast, HMMA AUC values in high-dose groups were <3-fold above those at 2 mg/kg. Several new findings emerge from this report of low-dose MDMA pharmacokinetics in rats. First, 2 mg/kg MDMA in rats can produce MDMA C(max) values similar to those in humans, perhaps explaining why both species discriminate 1.5 mg/kg MDMA in laboratory paradigms. Second, our data provide additional support for nonlinear kinetics of MDMA in rats, and, analogous to humans, this phenomenon appears to involve impaired drug metabolism. Finally, given key similarities between MDMA pharmacokinetics in rats and humans, data from rats may be clinically relevant when appropriate dosing conditions are used.
Figures
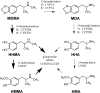
Fig. 1.
MDMA metabolism in humans and rats. Thick arrows represent major pathways of biotransformation, and thin arrows indicate minor pathways. The main cytochrome P450 isoforms responsible for specific reactions in humans (H) and rats (R) are noted. COMT, catechol-_O_-methyltransferase.
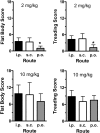
Fig. 2.
Effects of 2 and 10 mg/kg MDMA on 5-HT behavioral syndrome in rats. Rats bearing indwelling jugular catheters received MDMA by the intraperitoneal, subcutaneous, or oral route. Subjects were observed for 1 min immediately before the withdrawal of each blood specimen for the first 5 h after treatment (nine time points). Flattened body posture and forepaw treading were scored as described under Materials and Methods, and rats were given a single summed score for each behavior. Data are the mean ± S.D. for n = 4 to 5 rats/group. *, P < 0.05, compared with intraperitoneal and subcutaneous routes at 2 mg/kg MDMA.
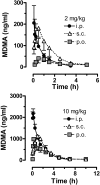
Fig. 3.
Time-concentration profiles for plasma MDMA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, or oral route. Rats bearing indwelling jugular catheters received MDMA at time 0, and blood specimens (0.25 ml) were withdrawn via catheters at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatment. Plasma was assayed for MDMA by GCMS as described under Materials and Methods. Data are mean ± S.D. for n = 4 to 5 rats/group.
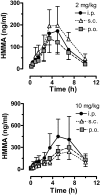
Fig. 4.
Time-concentration profiles for plasma HMMA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, or oral route. Rats bearing indwelling jugular catheters received MDMA at time 0, and blood specimens (0.25 ml) were withdrawn via catheters at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatment. Plasma was assayed for HMMA by GCMS as described under Materials and Methods. Data are the mean ± S.D. for n = 4 to 5 rats/group.
Fig. 5.
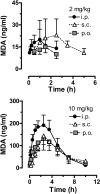
Time-concentration profiles for plasma MDA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, or oral route. Rats bearing indwelling jugular catheters received MDMA at time 0, and blood specimens (0.25 ml) were withdrawn via catheters at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatment. Plasma was assayed for MDA by GCMS as described under Materials and Methods. Data are the mean ± S.D. for n = 4 to 5 rats/group.
Fig. 6.
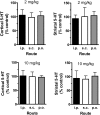
Effects of 2 and 10 mg/kg MDMA on postmortem tissue concentrations of 5-HT in rat cortex and striatum. Rats bearing indwelling jugular catheters received MDMA by the intraperitoneal, subcutaneous, or oral route. Subjects were returned to the vivarium after blood sampling procedures, and a group of control rats received 1 ml/kg i.p. saline. Two weeks later, all rats were decapitated, brain regions were dissected, and tissue was frozen until 5-HT assay by HPLC-electrochemical detection as described under Materials and Methods. Data are the mean ± S.D. for n = 4 to 5 rats/group expressed as a percentage of the saline-treated control. Tissue concentrations of 5-HT in control rats were 210 ± 52 and 319 ± 77 pg/mg of tissue for cortex and striatum, respectively.
Similar articles
- Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults.
Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Kolbrich EA, et al. Ther Drug Monit. 2008 Jun;30(3):320-32. doi: 10.1097/FTD.0b013e3181684fa0. Ther Drug Monit. 2008. PMID: 18520604 Free PMC article. Clinical Trial. - Nonlinear pharmacokinetics of (+/-)3,4-methylenedioxymethamphetamine (MDMA) and its pharmacodynamic consequences in the rat.
Concheiro M, Baumann MH, Scheidweiler KB, Rothman RB, Marrone GF, Huestis MA. Concheiro M, et al. Drug Metab Dispos. 2014 Jan;42(1):119-25. doi: 10.1124/dmd.113.053678. Epub 2013 Oct 18. Drug Metab Dispos. 2014. PMID: 24141857 Free PMC article. - 3,4-Methylenedioxymethamphetamine (MDMA) and metabolites disposition in blood and plasma following controlled oral administration.
Hartman RL, Desrosiers NA, Barnes AJ, Yun K, Scheidweiler KB, Kolbrich-Spargo EA, Gorelick DA, Goodwin RS, Huestis MA. Hartman RL, et al. Anal Bioanal Chem. 2014 Jan;406(2):587-99. doi: 10.1007/s00216-013-7468-y. Epub 2013 Nov 15. Anal Bioanal Chem. 2014. PMID: 24232751 Free PMC article. - Population pharmacokinetics of 3,4-methylenedioxymethamphetamine and main metabolites in rats.
Hirt D, Fonsart J, Menet MC, Debray M, Noble F, Declèves X, Scherrmann JM. Hirt D, et al. Toxicol Sci. 2010 Mar;114(1):38-47. doi: 10.1093/toxsci/kfp300. Epub 2009 Dec 14. Toxicol Sci. 2010. PMID: 20008456 - Distribution of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) stereoisomers in a fatal poisoning.
Moore KA, Mozayani A, Fierro MF, Poklis A. Moore KA, et al. Forensic Sci Int. 1996 Dec 2;83(2):111-9. doi: 10.1016/s0379-0738(96)02025-7. Forensic Sci Int. 1996. PMID: 9022274 Review.
Cited by
- Age differences in (±) 3,4-methylenedioxymethamphetamine (MDMA)-induced conditioned taste aversions and monoaminergic levels.
Cobuzzi JL, Siletti KA, Hurwitz ZE, Wetzell B, Baumann MH, Riley AL. Cobuzzi JL, et al. Dev Psychobiol. 2014 May;56(4):635-46. doi: 10.1002/dev.21132. Epub 2013 Jun 15. Dev Psychobiol. 2014. PMID: 23775255 Free PMC article. - A Multimodal Preclinical Assessment of MDMA in Female and Male Rats: Prohedonic, Cognition Disruptive, and Prosocial Effects.
Adam AS, LaMalfa KS, Razavi Y, Kohut SJ, Kangas BD. Adam AS, et al. Psychedelic Med (New Rochelle). 2024 Jun 17;2(2):96-108. doi: 10.1089/psymed.2023.0049. eCollection 2024 Jun. Psychedelic Med (New Rochelle). 2024. PMID: 39149579 - Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans.
Poyatos L, Lo Faro AF, Berardinelli D, Sprega G, Malaca S, Pichini S, Huestis MA, Papaseit E, Pérez-Mañá C, Busardò FP, Farré M. Poyatos L, et al. Int J Mol Sci. 2022 Nov 23;23(23):14636. doi: 10.3390/ijms232314636. Int J Mol Sci. 2022. PMID: 36498963 Free PMC article. Clinical Trial. - Environment Influencing Serotonin Syndrome Induced by Ecstasy Abuse.
Tao R, Shokry IM, Callanan JJ. Tao R, et al. Ann Forensic Res Anal. 2017;4(1):1039. Epub 2017 Mar 7. Ann Forensic Res Anal. 2017. PMID: 29732414 Free PMC article. - Effects of repeated 3,4-methylenedioxymethamphetamine administration on neurotransmitter efflux and sensory-evoked discharge in the ventral posterior medial thalamus.
Starr MA, Page ME, Waterhouse BD. Starr MA, et al. J Pharmacol Exp Ther. 2012 Jan;340(1):73-82. doi: 10.1124/jpet.111.185728. Epub 2011 Oct 7. J Pharmacol Exp Ther. 2012. PMID: 21984836 Free PMC article.
References
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. (2007) Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 35: 1840–1845 - PubMed
- Brady JF, Di Stefano EW, Cho AK. (1986) Spectral and inhibitory interactions of (±)-3,4-methylenedioxyamphetamine (MDA) and (±)-3,4-methylenedioxymethamphetamine (MDMA) with rat hepatic microsomes. Life Sci 39: 1457–1464 - PubMed
- Chu T, Kumagai Y, DiStefano EW, Cho AK. (1996) Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51: 789–796 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical