Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription - PubMed (original) (raw)
. 2009 Sep;11(9):1093-102.
doi: 10.1038/ncb1922. Epub 2009 Aug 16.
Affiliations
- PMID: 19684579
- PMCID: PMC6711162
- DOI: 10.1038/ncb1922
Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription
Diana A Stavreva et al. Nat Cell Biol. 2009 Sep.
Abstract
Studies on glucocorticoid receptor (GR) action typically assess gene responses by long-term stimulation with synthetic hormones. As corticosteroids are released from adrenal glands in a circadian and high-frequency (ultradian) mode, such treatments may not provide an accurate assessment of physiological hormone action. Here we demonstrate that ultradian hormone stimulation induces cyclic GR-mediated transcriptional regulation, or gene pulsing, both in cultured cells and in animal models. Equilibrium receptor-occupancy of regulatory elements precisely tracks the ligand pulses. Nascent RNA transcripts from GR-regulated genes are released in distinct quanta, demonstrating a profound difference between the transcriptional programs induced by ultradian and constant stimulation. Gene pulsing is driven by rapid GR exchange with response elements and by GR recycling through the chaperone machinery, which promotes GR activation and reactivation in response to the ultradian hormone release, thus coupling promoter activity to the naturally occurring fluctuations in hormone levels. The GR signalling pathway has been optimized for a prompt and timely response to fluctuations in hormone levels, indicating that biologically accurate regulation of gene targets by GR requires an ultradian mode of hormone stimulation.
Figures
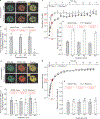
Figure 1
GR-MMTV promoter interactions in response to ultradian corticosterone fluctuations. (a) Corticosterone plasma levels in a freely moving rat show ultradian (pulsatile) fluctuations. (b) Pulsatile corticosterone treatment induces cyclic GR loading at the MMTV promoter array (green) in the 3617 cell line (single cell analysis). Scale bar, 2 μm. (c) GFP-GR intensity fluctuations at the array in response to the changing hormone concentration (in the cell presented in b). (d) GFP-GR dissociation from the array in response to hormone withdrawal (measured by the GFP-GR fluorescent intensity at the ChRFP-NF1-marked locus) is completed in < 10 min, whereas GR remains nuclear for > 3 h. Error bars represent the mean ± s.e.m., n = 25 cells. (e) Representative ChIP experiment demonstrates a similar rate of dissociation of endogenous GR from the MMTV promoter array on hormone withdrawal. (f) GFP-GR dissociation from the MMTV promoter is completed in <15 min after hormone withdrawal when the activating hormone is a naturally occurring glucocorticoid (corticosterone or hydrocortisone), but is incomplete for all of the synthetic glucocorticoids tested, indicating stronger and longer-lived interactions between the synthetic hormones and the GR. Error bars represent the mean ± s.e.m., n ≥ 300 cells.
Figure 2
GR-chaperone cycle. (a) GR is involved in frequent hormone disassociation-reassociation cycles. After hormone release, GR must reassociate with the chaperone machinery to regain its hormone-binding affinity in the nucleus. Hsp90 inhibition (with the selective Hsp90 inhibitor geldanamycin) in the constant presence of hormone (Cort), induces rapid GR disassociation from the array (within 5–10 min) before accelerated proteasome-mediated degradation. (b) Images of a single cell expressing GFP-GR and ChRFP-NF1. The cell was induced with corticosterone (100 nM), before being challenged with 2.5 μg ml−1 geldanamycin in the continuing presence of hormone. The equilibrium association of GFP-GR with the array decreases rapidly during inhibition, and total nuclear concentration of GR decreases afterwards due to accelerated degradation. The effect of geldanamycin treatment is confined to GR, as the intensity of the ChRFP-tagged NF1 at the array (and its concentration in the nucleus) is unaffected by it. Images were recorded at 5 min intervals. Geldanamycin was added 25 min after the start of the experiment, 20 min after corticosterone stimulation. Scale bar, 5 μm. (c) The average intensities (in relative units) of GFP-GR and ChRFP-NF1 associated with the array, or in the nucleus, are plotted as a function of time.
Figure 3
GFP-GR loading and dynamics at the MMTV promoter array during ultradian stimulation with natural and synthetic glucocorticoids. (a) A 3617 ChRFP-NF1 cell line was used to determine the GFP-GR loading at, and dissociation from, the MMTV array (marked by ChRFP-tagged NF1) during corticosterone induction (Pulse) and withdrawal (Washout). Scale bar, 5 μm. (b) 3617-ChRFP-NF1 cells were subjected to one, two or three cycles of 100 nM corticosterone induction (20 min) and withdrawal (40 min) and fixed either after the induction (P1, 2 and 3) or on hormone withdrawal (W1, 2 and 3). GR loading at the array (measured as GFP-GR intensity at the ChRFP-NF1-marked locus relative to the nucleoplasmic GFP-GR intensity) precisely followed the hormone addition and withdrawal cycles. Error bars represent the mean ± s.e.m., n = 9–14 cells. (c)GFP-GR dynamics at the array was measured by fluorescent recovery after photobleaching (FRAP) on corticosterone addition and withdrawal. Recovery during hormone stimulation, reflective of productive GR-MMTV promoter interactions, was slower than recovery on hormone withdrawal. Error bars represent the mean ± s.e.m., n ≥ 15 cells. (d)Similar fluctuations in GFP-GR dynamics were detected during subsequent cycles 2 and 3. Error bars represent the mean ± s.e.m., n ≥ 15 cells. (e) GFP-GR associates with the ChRFP-NFl-marked MMTV array after dexamethasone treatment, but remains associated with this locus even after dexamethasone removal from the growth media, indicating stable GR-dexamethasone interactions. Scale bar, 5 μm. (f) Only small fluctuations of the GFP-GR intensity at the array site are observed over three consecutive cycles of dexamethasone addition and withdrawals. Error bars represent the mean ± s.e.m., n = 17–29 cells. (g) A minor change in GFP-GR dynamics at the array is observed after dexamethasone washout. Fluorescent recovery 0.8 s after the bleach period is 65% ± 0.01 and 67% ± 0.02 for the pulsed and washout sample, respectively. Error bars represent the mean ± s.e.m., n ≥ 15 cells. (h) Comparable changes in the GFP-GR dynamics are observed during the second and third subsequent cycles. Error bars represent the mean ± s.e.m., n ≥ 15 cells.
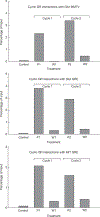
Figure 4
Equilibrium association of GR with endogenous GR response elements (GREs). GR associated with GREs was determined by chromatin immunoprecipitation (ChIP) during ultradian stimulation. Cyclic loading of GR is observed for the MMTV nucleosome B GR response element, the Glul response element, and the Mt1 response element. The data are from three independent experiments. P, induction; W, withdrawal.
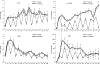
Figure 5
Cyclic RNA Polymerase II loading and dynamics in response to ultradian corticosterone treatment. (a) GFP-Pol II associates with the ChRFP-NF1-marked MMTV array locus in response to corticosterone induction (Pulse) and dissociates from the array on corticosterone withdrawal (Washout). Scale bar, 5 μm. (b) Cyclic Pol II loading at the MMTV promoter array, measured by GFP-Pol II intensity relative to nucleoplasmic intensity, during three consecutive cycles of hormone addition and withdrawal. Error bars represent the mean ± s.e.m., n = 8–15 cells. (c) GFP-Pol II dynamics at the array as measured by FRAP is slower during corticosterone induction, compared with withdrawal (54% and 76% recovery at 1.45 s after the bleach, respectively). Error bars represent the mean ± s.e.m., n ≥ 12 cells. Reduced Pol II mobility on hormone stimulation indicates that more Pol II molecules are productively engaged in transcription and elongation. (d) Comparable fluctuations in the GFP-Pol II dynamics are observed over several cycles of ultradian corticosterone treatment. P, induction; W, withdrawal. Error bars represent mean ± s.e.m., n = 12–19 cells.
Figure 6
Effects of ultradian corticosterone treatment on transcription of GR-regulated genes in 3134 cells. Nascent transcripts from several GR-upregulated genes in the 3134 cell line are synthesized in distinct quanta during the ultradian hormone induction (eight consecutive pulses), whereas constant corticosterone treatment induces continuous RNA release. Error bars represent mean ± s.e.m. from three independent experiments. C, control; P, induction; W, withdrawal.
Figure 7
Differential transcription outputs of ultradian and constant hormone treatments. (a) Two pulses of corticosterone induce characteristic pulsatile release of nascent RNA, whereas constant treatment induces continuous release of Per1 transcript. (b–d) Constant treatment leads to accumulation of higher levels of mature RNA from GR-regulated genes, Per1 (b), Mt2 (c) and Fkbp5 (d), than pulsatile treatment. Error bars represent the mean ± s.e.m. n = 4. (e) Western blot (WB) experiments demonstrate that the Fkbp5 protein level after constant treatment is higher than the protein level after pulsatile treatment (600 nM corticosterone for 8 h). Lane 1, pulsatile treatment; Lane 2, constant treatment.
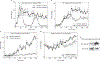
Figure 8
Pulsatile corticosterone treatment of adrenalectomized (ADX) rats and a model for gene pulsing. (a) Pulsatile corticosterone replacement in freely ambulating ADX rats was achieved with hourly bolus injections through an indwelling venous cannula. Plasma corticosterone levels, measured by an enzyme immunoassay (EIA), were undetectable in the ADX rats before corticosterone injection (time 0 min), and rose to a maximal value 1 min after injection before clearing according to the approximate 10 min half-life of corticosterone in blood (red trace). Subsequent injections at 60 min and 120 min resulted in similar profiles. GR activation and DNA association-dissociation kinetics reflected the pulsatile pattern of circulating corticosterone. GR activation and binding to GREs containing synthetic oligonucleotides were determined in a quantitative ELISA-based assay (TransAM) using nuclear extracts prepared from rat liver at the indicated times (blue trace). Chromatin immunoprecipitation (ChIP) assays (shown in Supplementary Information, Fig. S10) revealed GR association-dissociation with regulatory regions of the Period 1 gene promoter shows a similar profile to the results obtained in the TransAM assay. The functional output of this dynamic activation profile is the pulsatile release of nascent Per 1 RNA, measured by qPCR analysis of heterogeneous nuclear RNA (hnRNA) during the time course (green trace). Error bars represent mean ± s.e.m., n = 4–12 animals. (b) Gene pulsing: a schematic representation of cyclic GR interactions with response elements leading to pulsatile release of nascent RNA. Ultradian hormone fluctuations (periodicity ~1h) induce GR translocation to the nucleus, before transient interactions (timescale of seconds) with GREs (chromatin-binding cycle). In addition, GR is involved in the nuclear chaperone cycle (timescale of min), through which unliganded GR regains its hormone-binding affinity and re-enters the chromatin-binding cycle. Receptors recently disengaged from chromatin may lose ligand (i), or retain ligand (ii) and rebind chromatin. During hormone withdrawal periods, the unliganded GR fraction increases rapidly (timescale of min), leading to loss of GR from the array, faster GR mobility and transcription downregulation. Cort, ligand.
Comment in
- Steroid hormone pulsing drives cyclic gene expression.
Desvergne B, Héligon C. Desvergne B, et al. Nat Cell Biol. 2009 Sep;11(9):1051-3. doi: 10.1038/ncb0909-1051. Nat Cell Biol. 2009. PMID: 19724259
Similar articles
- Steroid hormone pulsing drives cyclic gene expression.
Desvergne B, Héligon C. Desvergne B, et al. Nat Cell Biol. 2009 Sep;11(9):1051-3. doi: 10.1038/ncb0909-1051. Nat Cell Biol. 2009. PMID: 19724259 - The HSP90 molecular chaperone cycle regulates cyclical transcriptional dynamics of the glucocorticoid receptor and its coregulatory molecules CBP/p300 during ultradian ligand treatment.
Conway-Campbell BL, George CL, Pooley JR, Knight DM, Norman MR, Hager GL, Lightman SL. Conway-Campbell BL, et al. Mol Endocrinol. 2011 Jun;25(6):944-54. doi: 10.1210/me.2010-0073. Epub 2011 Apr 21. Mol Endocrinol. 2011. PMID: 21511880 Free PMC article. - Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus.
Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, de Kloet ER, Lightman SL. Conway-Campbell BL, et al. J Neuroendocrinol. 2010 Oct;22(10):1093-1100. doi: 10.1111/j.1365-2826.2010.02051.x. J Neuroendocrinol. 2010. PMID: 20649850 Free PMC article. - Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis.
Wiley JW, Higgins GA, Athey BD. Wiley JW, et al. Neurogastroenterol Motil. 2016 Jan;28(1):12-25. doi: 10.1111/nmo.12706. Neurogastroenterol Motil. 2016. PMID: 26690871 Free PMC article. Review. - Circadian and ultradian patterns of HPA-axis activity in rodents: Significance for brain functionality.
den Boon FS, Sarabdjitsingh RA. den Boon FS, et al. Best Pract Res Clin Endocrinol Metab. 2017 Oct;31(5):445-457. doi: 10.1016/j.beem.2017.09.001. Epub 2017 Sep 20. Best Pract Res Clin Endocrinol Metab. 2017. PMID: 29223280 Review.
Cited by
- Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration.
Vyas S, Rodrigues AJ, Silva JM, Tronche F, Almeida OF, Sousa N, Sotiropoulos I. Vyas S, et al. Neural Plast. 2016;2016:6391686. doi: 10.1155/2016/6391686. Epub 2016 Mar 10. Neural Plast. 2016. PMID: 27034847 Free PMC article. Review. - Impairment of neutrophilic glucocorticoid receptor function in patients treated with steroids for septic shock.
Bergquist M, Lindholm C, Strinnholm M, Hedenstierna G, Rylander C. Bergquist M, et al. Intensive Care Med Exp. 2015 Dec;3(1):59. doi: 10.1186/s40635-015-0059-9. Epub 2015 Jul 28. Intensive Care Med Exp. 2015. PMID: 26215823 Free PMC article. - Synthetic biology: applications come of age.
Khalil AS, Collins JJ. Khalil AS, et al. Nat Rev Genet. 2010 May;11(5):367-79. doi: 10.1038/nrg2775. Nat Rev Genet. 2010. PMID: 20395970 Free PMC article. Review. - The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights.
Coutinho AE, Chapman KE. Coutinho AE, et al. Mol Cell Endocrinol. 2011 Mar 15;335(1):2-13. doi: 10.1016/j.mce.2010.04.005. Epub 2010 Apr 14. Mol Cell Endocrinol. 2011. PMID: 20398732 Free PMC article. Review. - Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males.
Russell GM, Henley DE, Leendertz J, Douthwaite JA, Wood SA, Stevens A, Woltersdorf WW, Peeters BW, Ruigt GS, White A, Veldhuis JD, Lightman SL. Russell GM, et al. J Neurosci. 2010 Apr 28;30(17):6106-15. doi: 10.1523/JNEUROSCI.5332-09.2010. J Neurosci. 2010. PMID: 20427668 Free PMC article. Clinical Trial.
References
- Lightman SL et al. Hypothalamic-pituitary-adrenal function. Arch. Physiol Biochem. 110, 90–93 (2002). - PubMed
- Lightman SL Patterns of exposure to glucocorticoid receptor ligand. Biochem. Soc. Trans. 34, 1117–1118 (2006). - PubMed
- Droste SK et al. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149, 3244–3253 (2008). - PubMed
- Young EA, Abelson J & Lightman SL Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 25, 69–76 (2004). - PubMed
- Lewis JG, Bagley CJ, Elder PA, Bachmann AW & Torpy DJ Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 359, 189–194 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 075548/Z/04/Z/WT_/Wellcome Trust/United Kingdom
- BB/C51297X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- ZIA BC010027-15/ImNIH/Intramural NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 074112/Z/04/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials