Stable transgene expression in primitive human CD34+ hematopoietic stem/progenitor cells, using the Sleeping Beauty transposon system - PubMed (original) (raw)
Stable transgene expression in primitive human CD34+ hematopoietic stem/progenitor cells, using the Sleeping Beauty transposon system
Teiko Sumiyoshi et al. Hum Gene Ther. 2009 Dec.
Abstract
Sleeping Beauty (SB) transposon-mediated integration has been shown to achieve long-term transgene expression in a wide range of host cells. In this study, we improved the SB transposon-mediated gene transfer system for transduction of human CD34(+) stem/progenitor cells by two approaches: (1) to increase the transposition efficacy, a hyperactive mutant of SB, HSB, was used; (2) to improve the expression of the SB transposase and the transgene cassette carried by the transposon, different viral and cellular promoters were evaluated. SB components were delivered in trans into the target cells by Nucleoporation. The SB transposon-mediated integration efficacy was assessed by integrated transgene (enhanced green fluorescent protein [eGFP]) expression both in vitro and in vivo. In purified human cord blood CD34(+) cells, HSB achieved long-term transgene expression in nearly 7-fold more cells than the original SB transposase. Significantly brighter levels of eGFP expression (5-fold) were achieved with the human elongation factor 1alpha (EF1-alpha) promoter in Jurkat human T cells, compared with that achieved with the modified myeloproliferative sarcoma virus long terminal repeat enhancer-promoter (MNDU3); in contrast, the MNDU3 promoter expressed eGFP at the highest level in K-562 myeloid cells. In human CD34(+) cord blood cells studied under conditions directing myeloid differentiation, the highest transgene integration and expression were achieved using the EF1-alpha promoter to express the SB transposase combined with the MNDU3 promoter to express the eGFP reporter. Stable transgene expression was achieved at levels up to 27% for more than 4 weeks of culture after improved gene transfer to CD34(+) cells (average, 17%; n = 4). In vivo studies evaluating engraftment and differentiation of the SB-modified human CD34(+) cells demonstrated that SB-modified human CD34(+) cells engrafted in NOD/SCID/gamma chain(null) (NSG) mice and differentiated into multilineage cell types with eGFP expression. More importantly, secondary transplantation studies demonstrated that the integrated transgene was stably expressed in more primitive CD34(+) hematopoietic stem cells (HSCs) with long-term repopulating capability. This study demonstrates that an improved HSB gene transfer system can stably integrate genes into primitive human HSCs while maintaining the pluripotency of the stem cells, which shows promise for further advancement of non-virus-based gene therapy using hematopoietic stem cells.
Figures
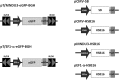
FIG. 1.
Vectors for _Sleeping Beauty_-mediated gene transfer. The pTransposon transposon plasmids include the Sleeping Beauty inverted/direct repeat (IR/DR) sequences flanking the expression cassette, which consists of the promoter, the enhanced green fluorescent protein (eGFP) coding sequence, and a bovine growth hormone (BGH) polyadenylation site (pA). The pSB transposase transient expression plasmids contain the Sleeping Beauty (SB) or hyperactive Sleeping Beauty (HSB16) transposase (or the mutant inactive SB, not shown) and the BGH poly(A) sequence driven by various viral and cellular promoters including human cytomegalovirus (CMV), the myeloproliferative sarcoma virus long terminal repeat enhancer–promoter (MNDU3), and human elongation factor 1α (EF1-α).
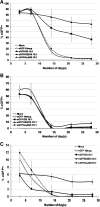
FIG. 2.
Stable long-term HSB- and SB-mediated gene transfer. Transgene expression over time in (A) K-562, (B) Jurkat, and (C) primary human CD34+ cells isolated from cord blood. Ten micrograms of the transposon plasmid (pT/MNDU3-eGFP-BGH) and 1 μg of either pCMV-SB (solid squares), pCMV-HSB (solid triangles), or pCMV-mutSB (solid circles) transposase-expressing plasmid were coelectroporated into each cell population. Cells were also electroporated with pT/MNDU3-eGFP-BGH alone (open squares) or with Amaxa Nucleoporation solution only, without added DNA (open diamonds), as controls. Aliquots of cells were analyzed by flow cytometry at each time point to determine the percentage of GFP-expressing cells and the percentage of propidium iodide (PI)+ cells. Each experiment was repeated three times (n = 3) with two or three replicates per experiment. Error bars represent the standard error of the mean (SEM).
FIG. 3.
Improvement of transposon-mediated gene transfer. (A) Viable frozen–thawed or freshly isolated human CD34+ cells (2 × 106) isolated from cord blood (CB) were electroporated with 10 μg of pT/MNDU3-eGFP-BGH with 1 μg of HSB transposase-expressing plasmid. Flow cytometric analysis was performed 4 weeks postelectroporation for stable transgene expression detection. Asterisks (* and **) indicate significant differences (p < 0.01; n = 3) between the data points. (B) Cells were electroporated with 10 μg of pT/MNDU3-eGFP-BGH according to either program U-08 or U-01 of the Amaxa Nucleofector. The percentages of eGFP+ cells (columns) and the percentages of PI+ cells (circles) were determined by flow cytometric analysis 3 days postelectroporation for transgene expression and cell viability, respectively. Asterisks (* and **) indicate significant differences (p < 0.05; n = 4) between the data points marked. (C) Primary human CD34+ cells were coelectroporated with 10 μg of pT/MNDU3-eGFP-BGH and increasing quantities of pCMV-SB (open columns), pCMV-HSB (solid columns), or pCMV-mutSB (gray columns). Flow cytometric analysis was performed 4 weeks postelectroporation for stable transgene expression (n = 2). (D) Ten micrograms of pT/MNDU3-eGFP-BGH was coelectroporated with increasing quantities of pCMV-SB (squares), pCMV-HSB (triangles), or pCMV-mutSB (circles) (n = 2). The percentages of PI+ cells were determined by flow cytometric analysis 3 days postelectroporation. Error bars represent the standard error of the mean (SEM).
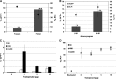
FIG. 4.
Promoter analysis for expression of transposase and transposon reporter. (A) Representative FACS plots showing stable HSB-mediated eGFP expression after 4 weeks in K-562, Jurkat, and primary human CD34+ cells (LTC-CD34+) cultured under conditions directing myeloid differentiation. Average mean fluorescence intensity (MFI) values are indicated in the top left corner of each plot. (B) Comparison of the average MFI of eGFP-expressing cells. LTC-CD34+ refers to long-term cultured human CD34+ cells under myeloid differentiation conditions. Ten micrograms of pT/MNDU3-eGFP-BGH or pT/EF1-α-eGFP-BGH transposon plasmid was coelectroporated with 1 μg of pEF1-α-HSB plasmid in this study. Cells were analyzed at week 4 postelectroporation for detection of HSB-mediated stable eGFP reporter gene expression. Each experiment was done with two or three replicates per condition. Error bars represent the SEM. The Student t test was performed for statistical analyses and p values are as indicated. *p < 0.01.
FIG. 5.
Overall SB transposon system improvement in primary human CD34+ hematopoietic progenitor/stem cells in vitro. Shown are representative FACS plots elucidating the progression of improvement of the SB-mediated gene integration system in this study. The percentages of eGFP-positive cells 4 weeks postelectroporation are indicated in the top left corner of each plot. The transposon–transposase plasmid DNA combinations used for each condition are also denoted at the top of the FACS plots.
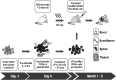
FIG. 6.
Schematic diagram of the experimental timeline for in vivo analysis of SB-mediated gene transfer to human CD34+ cells by NOD/SCID/γ chainnull neonatal transplantation. NSG, NOD/SCID/γ chainnull mouse.
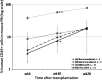
FIG. 7.
Comparison of engraftment levels of human CD34+ progenitor cells in NSG mice. At the indicated times after neonatal transplantation of human CD34+ cells, human CD45+ cells in mouse peripheral blood (PB) were assayed by flow cytometric analysis. Freshly isolated human CD34+ cells isolated from CB were coelectroporated with 10 μg of pT/MNDU3-eGFP-BGH and 1 μg of pEF1-α-HSB plasmid (group C; n = 11) or with 10 μg of pT/EF1-α-eGFP-BGH and 1 μg of pEF1-α-HSB plasmid (group D; n = 11). As study controls, NSG mice received human CD34+ cells that were not transduced (group A; n = 3) or that were “mock transduced” (electroporated without plasmid DNA) (group B; n = 4). Asterisks (* and **) indicate significant differences (p < 0.01) between the data points marked.
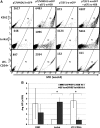
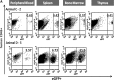
FIG. 8.
Representative flow cytometric analysis of eGFP-expressing human cells from NSG mice engrafted with HSB-modified human CD34+ cells at 5 months posttransplantation. (A) Stable eGFP expression was detected in peripheral blood, bone marrow, thymus, and spleen from NSG mice transplanted with human CD34+ cells modified by HSB-mediated gene transfer. (B) Stable eGFP expression was detected in CD4+ or CD8+ T cells, CD19+ B cells, CD56+ NK cells, and CD14+ myeloid cells from all organs. Data shown here are FACS plots of the BM and thymus harvested from C-2 and D-5 NSG mice. The thymus of mouse D-5 was not sufficiently populated for immunostaining analysis. (C) Total bone marrow cells harvested from NSG mouse D-5 were successfully engrafted into secondary adult NSG recipients. eGFP transgene expression was also detected in NSG human repopulation cells. Each number represents the percentages of human CD45+eGFP+ cells in the specific cell lineage.
FIG. 8.
Representative flow cytometric analysis of eGFP-expressing human cells from NSG mice engrafted with HSB-modified human CD34+ cells at 5 months posttransplantation. (A) Stable eGFP expression was detected in peripheral blood, bone marrow, thymus, and spleen from NSG mice transplanted with human CD34+ cells modified by HSB-mediated gene transfer. (B) Stable eGFP expression was detected in CD4+ or CD8+ T cells, CD19+ B cells, CD56+ NK cells, and CD14+ myeloid cells from all organs. Data shown here are FACS plots of the BM and thymus harvested from C-2 and D-5 NSG mice. The thymus of mouse D-5 was not sufficiently populated for immunostaining analysis. (C) Total bone marrow cells harvested from NSG mouse D-5 were successfully engrafted into secondary adult NSG recipients. eGFP transgene expression was also detected in NSG human repopulation cells. Each number represents the percentages of human CD45+eGFP+ cells in the specific cell lineage.
FIG. 8.
Representative flow cytometric analysis of eGFP-expressing human cells from NSG mice engrafted with HSB-modified human CD34+ cells at 5 months posttransplantation. (A) Stable eGFP expression was detected in peripheral blood, bone marrow, thymus, and spleen from NSG mice transplanted with human CD34+ cells modified by HSB-mediated gene transfer. (B) Stable eGFP expression was detected in CD4+ or CD8+ T cells, CD19+ B cells, CD56+ NK cells, and CD14+ myeloid cells from all organs. Data shown here are FACS plots of the BM and thymus harvested from C-2 and D-5 NSG mice. The thymus of mouse D-5 was not sufficiently populated for immunostaining analysis. (C) Total bone marrow cells harvested from NSG mouse D-5 were successfully engrafted into secondary adult NSG recipients. eGFP transgene expression was also detected in NSG human repopulation cells. Each number represents the percentages of human CD45+eGFP+ cells in the specific cell lineage.
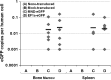
FIG. 9.
Summary of multilineage eGFP expression levels determined in peripheral blood, bone marrow, thymus, and spleen harvested from NSG mice 5 months after neonatal transplantation of HSB-modified human CD34+ cells. Shown is the percentage of human CD45+eGFP+ cells detected in CD4+ or CD8+ T cells, CD19+ B cells, CD56+ NK cells, and CD14+ myeloid cells from all organs harvested from (A) NSG mouse C-2 and (B) NSG mouse D-5.
FIG. 10.
Summary of eGFP gene marking in human CD45+ cells isolated from the BM and spleen of NSG mice 5 months posttransplantation. The geometric mean of the eGFP marking, calculated on the basis of the values listed in Table 3, is represented by the horizontal bars. Zero values were not included in the determination of geometric means.
Similar articles
- Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system.
Xue X, Huang X, Nodland SE, Mátés L, Ma L, Izsvák Z, Ivics Z, LeBien TW, McIvor RS, Wagner JE, Zhou X. Xue X, et al. Blood. 2009 Aug 13;114(7):1319-30. doi: 10.1182/blood-2009-03-210005. Epub 2009 May 4. Blood. 2009. PMID: 19414858 - Stable gene transfer to human CD34(+) hematopoietic cells using the Sleeping Beauty transposon.
Hollis RP, Nightingale SJ, Wang X, Pepper KA, Yu XJ, Barsky L, Crooks GM, Kohn DB. Hollis RP, et al. Exp Hematol. 2006 Oct;34(10):1333-43. doi: 10.1016/j.exphem.2006.05.023. Exp Hematol. 2006. PMID: 16982326 - High-level genomic integration, epigenetic changes, and expression of sleeping beauty transgene.
Zhu J, Park CW, Sjeklocha L, Kren BT, Steer CJ. Zhu J, et al. Biochemistry. 2010 Feb 23;49(7):1507-21. doi: 10.1021/bi9016846. Biochemistry. 2010. PMID: 20041635 Free PMC article. - Translating Sleeping Beauty transposition into cellular therapies: victories and challenges.
Izsvák Z, Hackett PB, Cooper LJ, Ivics Z. Izsvák Z, et al. Bioessays. 2010 Sep;32(9):756-67. doi: 10.1002/bies.201000027. Bioessays. 2010. PMID: 20652893 Free PMC article. Review. - Transposon-mediated gene transfer into adult and induced pluripotent stem cells.
Belay E, Dastidar S, VandenDriessche T, Chuah MK. Belay E, et al. Curr Gene Ther. 2011 Oct;11(5):406-13. doi: 10.2174/156652311797415836. Curr Gene Ther. 2011. PMID: 21864290 Review.
Cited by
- DNA transposon-based gene vehicles - scenes from an evolutionary drive.
Skipper KA, Andersen PR, Sharma N, Mikkelsen JG. Skipper KA, et al. J Biomed Sci. 2013 Dec 9;20(1):92. doi: 10.1186/1423-0127-20-92. J Biomed Sci. 2013. PMID: 24320156 Free PMC article. Review. - The Sleeping Beauty transposon system: a non-viral vector for gene therapy.
Aronovich EL, McIvor RS, Hackett PB. Aronovich EL, et al. Hum Mol Genet. 2011 Apr 15;20(R1):R14-20. doi: 10.1093/hmg/ddr140. Epub 2011 Apr 1. Hum Mol Genet. 2011. PMID: 21459777 Free PMC article. Review. - Alpharetroviral vector-mediated gene therapy for X-CGD: functional correction and lack of aberrant splicing.
Kaufmann KB, Brendel C, Suerth JD, Mueller-Kuller U, Chen-Wichmann L, Schwäble J, Pahujani S, Kunkel H, Schambach A, Baum C, Grez M. Kaufmann KB, et al. Mol Ther. 2013 Mar;21(3):648-61. doi: 10.1038/mt.2012.249. Epub 2012 Dec 4. Mol Ther. 2013. PMID: 23207695 Free PMC article. - The impact of cHS4 insulators on DNA transposon vector mobilization and silencing in retinal pigment epithelium cells.
Sharma N, Hollensen AK, Bak RO, Staunstrup NH, Schrøder LD, Mikkelsen JG. Sharma N, et al. PLoS One. 2012;7(10):e48421. doi: 10.1371/journal.pone.0048421. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110238 Free PMC article. - Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo.
Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC, Cannon PM. Holt N, et al. Nat Biotechnol. 2010 Aug;28(8):839-47. doi: 10.1038/nbt.1663. Epub 2010 Jul 2. Nat Biotechnol. 2010. PMID: 20601939 Free PMC article.
References
- Ailles L. Schmidt M. Santoni De Sio F.R. Glimm H. Cavalieri S. Bruno S. Piacibello W. Von Kalle C. Naldini L. Molecular evidence of lentiviral vector-mediated gene transfer into human self-renewing, multi-potent, long-term NOD/SCID repopulating hematopoietic cells. Mol. Ther. 2002;6:615–626. - PubMed
- Aiuti A. Slavin S. Aker M. Ficara F. Deola S. Mortellaro A. Morecki S. Andolfi G. Tabucchi A. Carlucci F. Marinello E. Cattaneo F. Vai S. Servida P. Miniero R. Roncarolo M.G. Bordignon C. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
- Baskar J.F. Smith P.P. Nilaver G. Jupp R.A. Hoffmann S. Peffer N.J. Tenney D.J. Colberg-Poley A.M. Ghazal P. Nelson J.A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J. Virol. 1996;70:3207–3214. - PMC - PubMed
- Baus J. Liu L. Heggestad A.D. Sanz S. Fletcher B.S. Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol. Ther. 2005;12:1148–1156. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical