Novel insights into adipogenesis from omics data - PubMed (original) (raw)
Review
Novel insights into adipogenesis from omics data
Andreas Prokesch et al. Curr Med Chem. 2009.
Free PMC article
Abstract
Obesity, the excess accumulation of adipose tissue, is one of the most pressing health problems in both the Western world and in developing countries. Adipose tissue growth results from two processes: the increase in number of adipocytes (hyperplasia) that develop from precursor cells, and the growth of individual fat cells (hypertrophy) due to incorporation of triglycerides. Adipogenesis, the process of fat cell development, has been extensively studied using various cell and animal models. While these studies pointed out a number of key factors involved in adipogenesis, the list of molecular components is far from complete. The advance of high-throughput technologies has sparked many experimental studies aimed at the identification of novel molecular components regulating adipogenesis. This paper examines the results of recent studies on adipogenesis using high-throughput technologies. Specifically, it provides an overview of studies employing microarrays for gene expression profiling and studies using gel based and non-gel based proteomics as well as a chromatin immunoprecipitation followed by microarray analysis (ChIP-chip) or sequencing (ChIP-seq). Due to the maturity of the technology, the bulk of the available data was generated using microarrays. Therefore these data sets were not only reviewed but also underwent meta analysis. The review also shows that large-scale omics technologies in conjunction with sophisticated bioinformatics analyses can provide not only a list of novel players, but also a global view on biological processes and molecular networks. Finally, developing technologies and computational challenges associated with the data analyses are highlighted, and an outlook on the questions not previously addressed is provided.
Figures
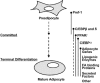
Fig. (1)
Development of mature adipocytes from preadipocytes. (Adopted from [119]). Dlk1 (Pref-1), Cebpb and Cebpd are expressed at the early stages of the differentiation process. After commitment, Pparg and Cebpa are upregulated and target many genes relevant for the function of mature adipocytes.
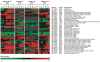
Fig. (2)
Gene expression profiles of selected candidates from 3T3-L1 time series experiments. 1Raw data were downloaded from Gene Expression Omnibus (GEO) GSE6794, normalized using GCRMA and annotated with recent annotation files for Mu11kA and Mu11kB downloaded from the Affymetrix website. Log2ratios are visualized for each time point in relation to the preconfluent stage; 2Normalized expression data from Ross et al. 2002 were downloaded from the authors’ website
http://wwwpersonal. umich.edu/~macdouga/MacDougaldLab.html
and data set were filtered for genes, which did not show an average difference of >100 in at least one condition. Relative expression levels (log2ratios) were related to the 3T3-L1 preconfluent stage and averaged over two measurements; 3Data for Hackl et al. 2005 was derived from ArrayExpress (E-MARS-02), normalized as stated by the authors and ESTs were annotated using a current Refseq version. Relative expression levels (log2ratios) from different time points were related to the preconfluent state and averaged over 3 independent experiments; 4Raw data (CEL files) were downloaded from
http://wwww.arcs.uams.edu/ directory/microarray.asp
, normalized using GCRMA and annotated using an annotation file provided by Affymetrix for U74Av2 mouse microarrays. Relative gene expressions were related to time point 0 immediately before hormonal induction and data were averaged over 2 replicated experiments; data were color coded according to the color scheme added to the figure).
Similar articles
- Autophagic Regulation of Adipogenesis Through TP53INP2: Insights from In Silico and In Vitro Analysis.
Sekar M, Thirumurugan K. Sekar M, et al. Mol Biotechnol. 2024 May;66(5):1188-1205. doi: 10.1007/s12033-023-01020-6. Epub 2024 Jan 18. Mol Biotechnol. 2024. PMID: 38238641 - Genome-wide profiling of transcription factor binding and epigenetic marks in adipocytes by ChIP-seq.
Nielsen R, Mandrup S. Nielsen R, et al. Methods Enzymol. 2014;537:261-79. doi: 10.1016/B978-0-12-411619-1.00014-8. Methods Enzymol. 2014. PMID: 24480351 - Characterization of ScAP-23, a new cell line from murine subcutaneous adipose tissue, identifies genes for the molecular definition of preadipocytes.
Kim JY, Wu Y, Smas CM. Kim JY, et al. Physiol Genomics. 2007 Oct 22;31(2):328-42. doi: 10.1152/physiolgenomics.00206.2006. Epub 2007 Jul 3. Physiol Genomics. 2007. PMID: 17609412 - Application of proteomics technology in adipocyte biology.
Renes J, Mariman E. Renes J, et al. Mol Biosyst. 2013 Jun;9(6):1076-91. doi: 10.1039/c3mb25596d. Epub 2013 Apr 29. Mol Biosyst. 2013. PMID: 23629546 Review. - Murine in vitro cellular models to better understand adipogenesis and its potential applications.
Vohra MS, Ahmad B, Serpell CJ, Parhar IS, Wong EH. Vohra MS, et al. Differentiation. 2020 Sep-Oct;115:62-84. doi: 10.1016/j.diff.2020.08.003. Epub 2020 Aug 23. Differentiation. 2020. PMID: 32891960 Review.
Cited by
- Dexamethasone and rosiglitazone are sufficient and necessary for producing functional adipocytes from mesenchymal stem cells.
Contador D, Ezquer F, Espinosa M, Arango-Rodriguez M, Puebla C, Sobrevia L, Conget P. Contador D, et al. Exp Biol Med (Maywood). 2015 Sep;240(9):1235-46. doi: 10.1177/1535370214566565. Epub 2015 Jan 16. Exp Biol Med (Maywood). 2015. PMID: 25595190 Free PMC article. - Adipogenesis: it is not just lipid that comprises adipose tissue.
Dodson MV, Jiang Z, Du M, Hausman GJ. Dodson MV, et al. J Genomics. 2013 Oct 1;1:1-4. doi: 10.7150/jgen.3276. eCollection 2013. J Genomics. 2013. PMID: 25031649 Free PMC article. - RNA-seq identified a super-long intergenic transcript functioning in adipogenesis.
Yi F, Yang F, Liu X, Chen H, Ji T, Jiang L, Wang X, Yang Z, Zhang LH, Ding X, Liang Z, Du Q. Yi F, et al. RNA Biol. 2013 Jun;10(6):991-1001. doi: 10.4161/rna.24644. Epub 2013 Apr 11. RNA Biol. 2013. PMID: 23603976 Free PMC article. - α/β-hydrolase domain containing protein 15 (ABHD15)--an adipogenic protein protecting from apoptosis.
Walenta E, Pessentheiner AR, Pelzmann HJ, Deutsch A, Goeritzer M, Kratky D, Hackl H, Oh DY, Prokesch A, Bogner-Strauss JG. Walenta E, et al. PLoS One. 2013 Nov 13;8(11):e79134. doi: 10.1371/journal.pone.0079134. eCollection 2013. PLoS One. 2013. PMID: 24236098 Free PMC article. - Mouse Embryonic Fibroblast Adipogenic Potential: A Comprehensive Transcriptome Analysis.
Al-Sayegh M, Ali H, Jamal MH, ElGindi M, Chanyong T, Al-Awadi K, Abu-Farha M. Al-Sayegh M, et al. Adipocyte. 2021 Dec;10(1):1-20. doi: 10.1080/21623945.2020.1859789. Adipocyte. 2021. PMID: 33345692 Free PMC article.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. - PubMed
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources