Overexpression of Rheb2 enhances mouse hematopoietic progenitor cell growth while impairing stem cell repopulation - PubMed (original) (raw)
Overexpression of Rheb2 enhances mouse hematopoietic progenitor cell growth while impairing stem cell repopulation
Timothy B Campbell et al. Blood. 2009.
Abstract
Molecular mechanisms preserving hematopoietic stem cell (HSC) self-renewal by maintaining a balance between proliferation, differentiation, and other processes are not fully understood. Hyperactivation of the mammalian target of rapamycin (mTOR) pathway, causing sustained proliferative signals, can lead to exhaustion of HSC repopulating ability. We examined the role of the novel ras gene Rheb2, an activator of the mTOR kinase, in colony-forming ability, survival, and repopulation of immature mouse hematopoietic cells. In a cell line model of mouse hematopoietic progenitor cells (HPCs), we found enhanced proliferation and mTOR signaling in cells overexpressing Rheb2. In addition, overexpression of Rheb2 enhanced colony-forming ability and survival of primary mouse bone marrow HPCs. Expansion of phenotypic HSCs in vitro was enhanced by Rheb2 overexpression. Consistent with these findings, Rheb2 overexpression transiently expanded phenotypically defined immature hematopoietic cells after in vivo transplantation; however, these Rheb2-transduced cells were significantly impaired in overall repopulation of primary and secondary congenic transplantation recipients. Our findings suggest that HPCs and HSCs behave differently in response to growth-promoting signals stimulated by Rheb2. These results may have value in elucidating mechanisms controlling the balance between proliferation and repopulating ability, a finding of importance in clinical uses of HPCs/HSCs.
Figures
Figure 1
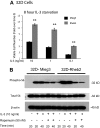
Rheb2 overexpression enhanced IL-3 proliferation and mTOR signaling in a mouse hematopoietic progenitor cell line. (A) Proliferation of transduced 32D cells in response to delayed IL-3 addition (combined data from 2 independent experiments each done in triplicate; mean ± SEM). **P < .01. (B) Western blot of 32D cell lysates after delayed addition of IL-3 ± rapamycin. Total S6 protein and β-actin are shown as loading controls. Western blot is representative of 2 independent experiments.
Figure 2
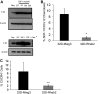
Rheb2 overexpression enhanced primary mouse hematopoietic progenitor colony formation and survival. (A) Real-time PCR expression of Rheb2 in FACS-sorted subsets of primary mBM cells, 2 or 3 replicates per cell type. (B) Numbers of CFU-GM and CFU-GEMM colonies produced by Mieg3- and Rheb2-transduced mBM cells (3 combined independent experiments, each done in triplicate; mean ± SEM). *P < .05. (C) Numbers of CFU-GM colonies produced in duplicate expanded liquid cultures (mean ± SD). *P < .05. (D) Survival of Mieg3- and Rheb2-transduced mBM cells in delayed growth factor colony assays, representative of 2 experiments each done in triplicate (mean ± SD).
Figure 3
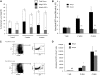
Overexpression of Rheb2 impairs SDF-1/CXCL12 chemotaxis and CXCR4 expression in 32D cells. (A) Activation of mTOR signaling in response to SDF-1/CXCL12 stimulation in mBM cells, representative of 2 independent experiments. (Bottom panel) Effect of rapamycin at reversing phosphorylation of S6, 100 ng/mL SDF-1/CXCL12 used in this experiment. (B) Transwell chemotaxis of transduced 32D cells to 100 ng/mL SDF-1/CXCL12, combined triplicates from 2 independent experiments. (C) 32D cells, FACS stained for CXCR4 surface expression, combined 2 independent experiments. *P < .02. **P = .05.
Figure 4
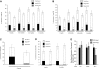
Rheb2 overexpression expanded phenotypic HSC (KSL) cells in vitro and increased cell cycling. (A) Representative FACS plots of KSL cells in cultures of Rheb2- and Mieg3-transduced mBM cells. (B) Tabulated data from 2 experiments, each done in duplicate 4 days after transduction (mean ± SEM). (C-D) Representative histograms showing cell cycling of Mieg3- and Rheb2-transduced KSL cells and tabulated data from duplicate cultures; mean ± SD. *P < .05. Rheb2 GFP+ fraction compared with Mieg3 GFP+ fraction in panel D.
Figure 5
Rheb2 overexpression impaired total donor cell engraftment but transiently expanded phenotypic stem/progenitor cells in vivo in short-term competitive repopulation experiments. (A) PB chimerism of transduced GFP+ donor (CD45.2+) cells and total donor (CD45.2+) cells in recipient animals (at least 5 animals in each group at each time point; mean ± SEM). (B) Chimerism of absolute numbers of transduced GFP+ donor cells (CD45.2+) in the BM of recipients (at least 5 animals in each group at each time point; mean ± SEM). (C) Representative FACS plots showing the percentage of GFP+ cells within the immature LS population in recipient animals at 2 weeks after transplantation. (D) The absolute numbers of GFP+LS cells in the BM of recipients (at least 5 animals in each group at each time point; mean ± SEM). *P < .05. **P < .01. NS indicates not significant.
Figure 6
Overexpression of Rheb2 decreased long-term competitive repopulation in vivo. (A) PB chimerism of transduced GFP+ donor (CD45.2+) cells and total donor (CD45.2+) cells in the 1:1 transplantation group (2 independent experiments, at least 11 animals in each group; mean ± SEM). (B) PB chimerism of transduced GFP+ donor (CD45.2+) cells and total donor (CD45.2+) cells in the 0.4:1 transplantation group (2 independent experiments, at least 11 animals in each group; mean ± SEM). (C) Primary recipient BM chimerism of transduced GFP+ donor (CD45.2+) cells and total donor (CD45.2+) cells in 1:1 group from one of the experiments in panel A (6 Mieg3 and 5 Rheb2 animals; mean ± SEM). (D) PB chimerism of transduced GFP+ donor (CD45.2+) cells and total donor (CD45.2+) cells in secondary transplantation recipients (4 secondary recipients per primary animal; mean ± SEM). *P < .05. **P < .02. NS indicates not significant. (E) Real-time PCR analysis of p21, PTEN, and Gfi-1 gene expression in transduced 32D cells. HPRT was used as internal control to standardize expression of all genes in both cell lines. Data are expressed as fold change over levels in 32D-Mieg3 cells, p21 and Gfi-1 are combined results from 3 experiments, each done at least in duplicate, whereas PTEN is combined results from 2 experiments, each done at least in duplicate. *P < .05. **P < .02.
Similar articles
- Enhancing engraftment of cord blood cells via insight into the biology of stem/progenitor cell function.
Broxmeyer HE. Broxmeyer HE. Ann N Y Acad Sci. 2012 Aug;1266(1):151-60. doi: 10.1111/j.1749-6632.2012.06509.x. Ann N Y Acad Sci. 2012. PMID: 22901266 Free PMC article. Review. - Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence.
Luo Y, Li L, Zou P, Wang J, Shao L, Zhou D, Liu L. Luo Y, et al. Transplantation. 2014 Jan 15;97(1):20-9. doi: 10.1097/TP.0b013e3182a7fcf8. Transplantation. 2014. PMID: 24092377 Free PMC article. - Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice.
Huang J, Zhang Y, Bersenev A, O'Brien WT, Tong W, Emerson SG, Klein PS. Huang J, et al. J Clin Invest. 2009 Dec;119(12):3519-29. doi: 10.1172/JCI40572. J Clin Invest. 2009. PMID: 19959876 Free PMC article. - Co-cultured hBMSCs and HUVECs on human bio-derived bone scaffolds provide support for the long-term ex vivo culture of HSC/HPCs.
Huang X, Li C, Zhu B, Wang H, Luo X, Wei L. Huang X, et al. J Biomed Mater Res A. 2016 May;104(5):1221-30. doi: 10.1002/jbm.a.35656. Epub 2016 Feb 12. J Biomed Mater Res A. 2016. PMID: 26779960 - Role of the mammalian target of rapamycin pathway in lentiviral vector transduction of hematopoietic stem cells.
Wang CX, Torbett BE. Wang CX, et al. Curr Opin Hematol. 2015 Jul;22(4):302-8. doi: 10.1097/MOH.0000000000000150. Curr Opin Hematol. 2015. PMID: 26049750 Free PMC article. Review.
Cited by
- Transient mTOR inhibition facilitates continuous growth of liver tumors by modulating the maintenance of CD133+ cell populations.
Yang Z, Zhang L, Ma A, Liu L, Li J, Gu J, Liu Y. Yang Z, et al. PLoS One. 2011;6(12):e28405. doi: 10.1371/journal.pone.0028405. Epub 2011 Dec 1. PLoS One. 2011. PMID: 22145042 Free PMC article. - Loss of Nupr1 promotes engraftment by tuning the quiescence threshold of hematopoietic stem cell repository via regulating p53-checkpoint pathway.
Wang T, Xia C, Weng Q, Wang K, Dong Y, Hao S, Dong F, Liu X, Liu L, Geng Y, Guan Y, Du J, Cheng T, Cheng H, Wang J. Wang T, et al. Haematologica. 2022 Jan 1;107(1):154-166. doi: 10.3324/haematol.2019.239186. Haematologica. 2022. PMID: 33299232 Free PMC article. - Enhancing engraftment of cord blood cells via insight into the biology of stem/progenitor cell function.
Broxmeyer HE. Broxmeyer HE. Ann N Y Acad Sci. 2012 Aug;1266(1):151-60. doi: 10.1111/j.1749-6632.2012.06509.x. Ann N Y Acad Sci. 2012. PMID: 22901266 Free PMC article. Review. - mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis.
Kalaitzidis D, Sykes SM, Wang Z, Punt N, Tang Y, Ragu C, Sinha AU, Lane SW, Souza AL, Clish CB, Anastasiou D, Gilliland DG, Scadden DT, Guertin DA, Armstrong SA. Kalaitzidis D, et al. Cell Stem Cell. 2012 Sep 7;11(3):429-39. doi: 10.1016/j.stem.2012.06.009. Cell Stem Cell. 2012. PMID: 22958934 Free PMC article. - Efficacy of RNA polymerase II inhibitors in targeting dormant leukaemia cells.
Pallis M, Burrows F, Whittall A, Boddy N, Seedhouse C, Russell N. Pallis M, et al. BMC Pharmacol Toxicol. 2013 Jun 15;14:32. doi: 10.1186/2050-6511-14-32. BMC Pharmacol Toxicol. 2013. PMID: 23767415 Free PMC article.
References
- Shen SW, Dolnikov A, Passioura T, et al. Mutant N-ras preferentially drives human CD34+ hematopoietic progenitor cells into myeloid differentiation and proliferation both in vitro and in the NOD/SCID mouse. Exp Hematol. 2004;32(9):852–860. - PubMed
- Tuveson DA, Shaw AT, Willis NA, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5(4):375–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK7519/DK/NIDDK NIH HHS/United States
- R01 HL56416/HL/NHLBI NIH HHS/United States
- P01 HL053586/HL/NHLBI NIH HHS/United States
- R01 HL67384/HL/NHLBI NIH HHS/United States
- P01 HL53586/HL/NHLBI NIH HHS/United States
- R01 HL067384/HL/NHLBI NIH HHS/United States
- R01 HL056416/HL/NHLBI NIH HHS/United States
- T32 DK007519/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous