Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer's disease - PubMed (original) (raw)
Case Reports
Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer's disease
Rebecca F Rosen et al. Acta Neuropathol. 2010 Feb.
Abstract
Radiolabeled Pittsburgh compound B (PIB) is a benzothiazole imaging agent that usually binds with high affinity, specificity, and stoichiometry to cerebral beta-amyloid (Abeta) in patients with Alzheimer's disease. Among a cohort of ten AD subjects examined postmortem, we describe a case of idiopathic, end-stage Alzheimer's disease with heavy Abeta deposition yet substantially diminished high-affinity binding of (3)H-PIB to cortical homogenates and unfixed cryosections. Cortical tissue samples were analyzed by immunohistochemistry, electron microscopy, ELISA, immunoblotting, MALDI-TOF mass spectrometry, in vitro (3)H-PIB binding and (3)H-PIB autoradiography. The PIB-refractory subject met the histopathological criteria for AD. However, cortical tissue from this case contained more vascular beta-amyloidosis, higher levels of insoluble Abeta40 and Abeta42, and a higher ratio of Abeta40:Abeta42 than did tissue from the nine comparison AD cases. Furthermore, cerebral Abeta from the PIB-refractory subject displayed an unusual distribution of low- and high-molecular weight Abeta oligomers, as well as a distinct pattern of N- and C-terminally truncated Abeta peptides in both the soluble and insoluble cortical extracts. Genetically, the patient was apolipoprotein-E3/4 heterozygous, and exhibited no known AD-associated mutations in the genes for the beta-amyloid precursor protein, presenilin1 or presenilin2. Our findings suggest that PIB may differentially recognize polymorphic forms of multimeric Abeta in humans with Alzheimer's disease. In addition, while the prevalence of PIB-refractory cases in the general AD population remains to be determined, the paucity of high-affinity binding sites in this AD case cautions that minimal PIB retention in positron-emission tomography scans of demented patients may not always rule out the presence of Alzheimer-type Abeta pathology.
Figures
Fig. 1. Aβ and tau pathology in the superior temporal gyrus of the PIB-refractory Alzheimer’s case AD1
(a) Antibody 6E10-immunoreactive senile plaques (two denoted by arrows) and large-vessel CAA (black arrowhead). (b) Antibody CP13-immunoreactive, intra-somatal neurofibrillary tangles (two denoted by arrowheads) and abundant neuropil threads. (c) Ultrastructure of amyloid fibrils in the periphery of a senile plaque. (d) Ultrastructure of a bundle of paired helical filaments (marked by two arrowheads). Case AD1 had significant CAA, and the senile plaques sometimes showed diminished immunostaining in the central region (right-hand arrow in a), but otherwise the histopathology did not differ remarkably from that of the other AD cases. Bar = 100μm in a and b and 200nm in c and d).
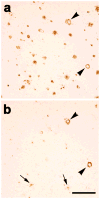
Fig. 2. Cerebral β-amyloid angiopathy in the occipital cortex of case AD1
Both large vessels (black arrowheads) and capillaries (grey arrowhead) were affected. CAA of both types was more abundant in the occipital cortex than in the temporal cortex. A plaque-like parenchymal deposit of Aβ is denoted by the arrow. Antibody 4G8; Bar = 200μm.
Fig. 3. Immunostaining for Aβ42 (a) and Aβ40 (b) in nearby temporal cortical sections from case AD1
The antibody to Aβ42 stained a variety of diffuse and dense parenchymal lesions as well as CAA, but the antibody to Aβ40 stained mainly CAA (the same two immunoreactive blood vessels are marked by arrowheads in both panels) and dense plaque cores (two denoted by arrows in b). Bar = 300μm.
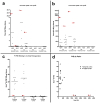
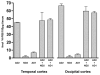
Fig. 4. Aβ levels and 3H-PIB binding in temporal and occipital cortical homogenates from AD1, nine comparison AD cases and three nondemented control (ND) cases
(a, b) By ELISA of soluble and insoluble extracts, AD1 (red arrowheads) exhibited substantially more soluble and insoluble Aβ40 and Aβ42 than did all other AD cases examined in this study. Negligible Aβ was detected in both regions of the 3 ND cases (group means are indicated by the horizontal lines). Levels of soluble Aβ40 and Aβ42 (a) and levels of insoluble Aβ40 and Aβ42 (b) are expressed in fmol Aβ per 100μg wet tissue for each subject. (c) Radioligand binding assays with 3H-PIB reveal high levels of PIB binding to temporal and occipital cortical tissue from all AD cases in this study except AD1, which showed only background levels of PIB binding. (d) The ratio of PIB binding to total Aβ in two cortical regions. When AD1 data points were excluded from the analysis, total levels of insoluble Aβ (Aβ40 and Aβ42) correlate positively with 3H-PIB binding to AD temporal (_n_=9) and occipital (_n_=7) cortical homogenates from the comparison AD cases [29].
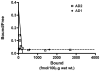
Fig. 5. Analysis of high-affinity 3H-PIB binding in temporal cortical homogenates from two AD cases
A homologous competition binding analysis of PIB binding levels in temporal cortical homogenates incubated with 1.2nM 3H-PIB alone and with 1.2nM 3H-PIB co-incubated with concentrations of unlabeled PIB between 1.0 nM and 1.0μM did not reveal a measurable high-affinity PIB binding component in case AD1 (dotted line). In case AD2 (continuous line), a high-affinity PIB binding component was detected with a Kd = 3.0nM and Bmax = 209.28 fmol/100μg wet tissue (AD2: open squares, AD1: filled diamonds). Comparable results were obtained with occipital cortical homogenates (data not shown).
Fig. 6. 3H-PIB binding experiments with mixtures of AD and ND cortical homogenates
To detect the potential presence of a diffusible element in AD1 cortical tissue that inhibits PIB binding in vitro, we incubated 1.0nM 3H-PIB with 1:1 mixtures of cortical homogenates from a typical PIB-sensitive AD case (AD2) and the PIB-refractory case AD1. We did not identify an inhibitory or synergistic effect on PIB binding in either the temporal or occipital cortical homogenates. Rather, the quantity of PIB binding to mixed homogenates was approximately the sum of total PIB binding to each individual homogenate. Similar results were seen when 1.0nM 3H-PIB was incubated with mixtures of temporal and occipital cortical homogenate from AD2 and a nondemented control case (ND2) that alone exhibited negligible high-affinity PIB binding.
Fig. 7. 3H-PIB Autoradiography in temporal cortical sections from case AD1 and a PIB-sensitive AD case (AD5)
(a) At a 1.0nM concentration, 3H-PIB binds to few cortical plaques and lightly to some blood vessels in the superior temporal cortex of AD1. (b) In an adjacent cryosection, immunohistochemistry with the 6E10 antibody to Aβ reveals abundant senile plaques as well as CAA in this cortical region. The blue arrowheads denote PIB-positive Aβ plaques and CAA, although the greater part of the Aβ-immunoreactive plaques and vessels did not bind to PIB by autoradiography. (c) In case AD5, 3H-PIB autoradiography and (d) 6E10 immunohistochemistry on adjacent cryosections demonstrate heavy, PIB-positive senile plaque deposition in the superior temporal cortex. Red arrows indicate PIB-positive Aβ lesions identified in adjacent cryosections.
Fig. 8. Western blot analysis of multimeric Aβ and APP in AD temporal cortex
Temporal cortical homogenates containing 60μg of total protein from case AD1 and seven other AD cases were separated by SDS-PAGE and immunoblotted with antibody 6E10 to the N-terminal region of Aβ. A preparation of synthetic Aβ42 (10 ng) is in the far right lane, as a positive control. Strong bands of monomeric Aβ were detected in all AD cases examined. Light bands corresponding to Aβ dimers were detected only in some of the AD lanes under these conditions. In AD1, 6E10 immunoblotting revealed high levels of dimeric and trimeric Aβ, as well as several higher molecular weight, Aβ-immunoreactive bands between 17 KDa and 44KDa. Note that 6E10 also recognizes the Aβ epitope in APP (top band), which was not increased in AD1. When the total protein content in AD1 temporal cortex was diluted to 6.78μg to normalize total Aβ levels to those seen in the comparison AD cases, the higher molecular weight bands, though lighter, were still detected by 6E10 immunoblot analysis, indicating that the presence of these bands cannot be accounted for simply by the overall quantity of Aβ in AD1 temporal cortex (data not shown).
Fig. 9. Immunoprecipitation/MALDI-TOF MS detection of Aβ peptides in the temporal cortex of case AD1 and a comparison PIB-sensitive AD case (AD12)
By mass spectrometry, we detected substantially more individual Aβ fragments in all cortical fractions of AD1 temporal cortex, as compared to three other AD cases (data from one comparison AD case shown). Numerous C-terminally truncated peptides (Aβ1-x) were seen in the buffer (TBS)-soluble extract, yet Aβ42 was barely detectable. These same truncated peptides also were detected in SDS-soluble extract, along with Aβ40 and Aβ42. N-terminally truncated peptides (Aβx-40 and Aβx-42) and full-length Aβ40 and Aβ42 predominated in formic acid-solubilized fractions (FA 70% and FA 100%) from AD1 cortex. This pattern of terminal-specific truncations in soluble and insoluble cortical fractions was not seen in the comparison AD cases or in a nondemented control case (not shown). The Aβ fragments written in red were particularly abundant, while those in parentheses were just barely detectable. a-42: Immunoprecipitation with monoclonal antibody 12F4, specific to Aβx-42; 4G8/6E10: Immunoprecipitation with two monoclonal antibodies, one to Aβ17–24 (4G8) and one to Aβ1–16 (6E10); TBS: Tris-buffered saline extract; SDS: sodium dodecyl sulfate-soluble extract; FA: formic acid-solubilized extract.
Similar articles
- Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease.
Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Ikonomovic MD, et al. Brain. 2008 Jun;131(Pt 6):1630-45. doi: 10.1093/brain/awn016. Epub 2008 Mar 12. Brain. 2008. PMID: 18339640 Free PMC article. - Post-mortem analyses of PiB and flutemetamol in diffuse and cored amyloid-β plaques in Alzheimer's disease.
Ikonomovic MD, Buckley CJ, Abrahamson EE, Kofler JK, Mathis CA, Klunk WE, Farrar G. Ikonomovic MD, et al. Acta Neuropathol. 2020 Oct;140(4):463-476. doi: 10.1007/s00401-020-02175-1. Epub 2020 Aug 9. Acta Neuropathol. 2020. PMID: 32772265 Free PMC article. - Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain.
Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, Debnath ML, Holt DP, Huang GF, Shao L, DeKosky ST, Price JC, Mathis CA. Klunk WE, et al. J Neurosci. 2005 Nov 16;25(46):10598-606. doi: 10.1523/JNEUROSCI.2990-05.2005. J Neurosci. 2005. PMID: 16291932 Free PMC article. - Positron emission tomography radiopharmaceuticals for imaging brain Beta-amyloid.
Vallabhajosula S. Vallabhajosula S. Semin Nucl Med. 2011 Jul;41(4):283-99. doi: 10.1053/j.semnuclmed.2011.02.005. Semin Nucl Med. 2011. PMID: 21624562 Review. - Relationship between memory performance and β-amyloid deposition at different stages of Alzheimer's disease.
Chételat G, Villemagne VL, Pike KE, Ellis KA, Ames D, Masters CL, Rowe CC; Australian Imaging Biomarkers and Lifestyle Study of Ageing Research Group. Chételat G, et al. Neurodegener Dis. 2012;10(1-4):141-4. doi: 10.1159/000334295. Epub 2012 Feb 1. Neurodegener Dis. 2012. PMID: 22301812 Review.
Cited by
- Context dependence of protein misfolding and structural strains in neurodegenerative diseases.
Mehta AK, Rosen RF, Childers WS, Gehman JD, Walker LC, Lynn DG. Mehta AK, et al. Biopolymers. 2013 Nov;100(6):722-30. doi: 10.1002/bip.22283. Biopolymers. 2013. PMID: 23893572 Free PMC article. - Automated detection of amyloid-β-related cortical and subcortical signal changes in a transgenic model of Alzheimer's disease using high-field MRI.
Teipel SJ, Kaza E, Hadlich S, Bauer A, Brüning T, Plath AS, Krohn M, Scheffler K, Walker LC, Lotze M, Pahnke J. Teipel SJ, et al. J Alzheimers Dis. 2011;23(2):221-37. doi: 10.3233/JAD-2010-101035. J Alzheimers Dis. 2011. PMID: 20966552 Free PMC article. - The benefits and limitations of animal models for translational research in neurodegenerative diseases.
Jucker M. Jucker M. Nat Med. 2010 Nov;16(11):1210-4. doi: 10.1038/nm.2224. Epub 2010 Sep 21. Nat Med. 2010. PMID: 21052075 - Emerging biomarkers in cognition.
Wicklund M, Petersen RC. Wicklund M, et al. Clin Geriatr Med. 2013 Nov;29(4):809-28. doi: 10.1016/j.cger.2013.07.006. Clin Geriatr Med. 2013. PMID: 24094298 Free PMC article. Review. - Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden.
Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O'Neil JP, Janabi M, Baker SL, Agarwal N, Bonasera SJ, Mormino EC, Weiner MW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ. Rabinovici GD, et al. Brain. 2010 Feb;133(Pt 2):512-28. doi: 10.1093/brain/awp326. Epub 2010 Jan 15. Brain. 2010. PMID: 20080878 Free PMC article.
References
- Attems J, Jellinger KA. Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology--a pilot study. Acta Neuropathol. 2004;107:83–90. - PubMed
- Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. - PubMed
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. - PubMed
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. - PubMed
- Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AG026423/AG/NIA NIH HHS/United States
- P50 AG025688-05/AG/NIA NIH HHS/United States
- R01 AG030539-02/AG/NIA NIH HHS/United States
- AG030539/AG/NIA NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P51 RR000165-498468/RR/NCRR NIH HHS/United States
- P50AG025688/AG/NIA NIH HHS/United States
- P51 RR000165-47/RR/NCRR NIH HHS/United States
- P01 AG026423/AG/NIA NIH HHS/United States
- RR-00165/RR/NCRR NIH HHS/United States
- P50 AG025688-01/AG/NIA NIH HHS/United States
- R01 AG030539/AG/NIA NIH HHS/United States
- P01 AG026423-03/AG/NIA NIH HHS/United States
- P51 RR000165-46/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous