Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry - PubMed (original) (raw)
Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry
Zihao Wang et al. Mol Cell Proteomics. 2010 Jan.
Abstract
Numerous cellular processes are regulated by the reversible addition of either phosphate or O-linked beta-N-acetylglucosamine (O-GlcNAc) to nuclear and cytoplasmic proteins. Although sensitive methods exist for the enrichment and identification of protein phosphorylation sites, those for the enrichment of O-GlcNAc-containing peptides are lacking. Reported here is highly efficient methodology for the enrichment and characterization of O-GlcNAc sites from complex samples. In this method, O-GlcNAc-modified peptides are tagged with a novel biotinylation reagent, enriched by affinity chromatography, released from the solid support by photochemical cleavage, and analyzed by electron transfer dissociation mass spectrometry. Using this strategy, eight O-GlcNAc sites were mapped from a tau-enriched sample from rat brain. Sites of GlcNAcylation were characterized on important neuronal proteins such as tau, synucleins, and methyl CpG-binding protein 2.
Figures
Fig. 1.
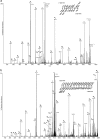
Enrichment of _O_-GlcNAc-modified peptides by a combination of enzymatic labeling with UDP-GalNAz and chemical derivatization with the photocleavable reagent PC-PEG-biotin-alkyne. a, flow chart showing the overall strategy. Inset, structure of the photocleavable PC-PEG-biotin-alkyne reagent. CIP, calf intestine phosphatase; PNGase F, peptide:_N_-Glycosidase F; TBTA, tris[(1-benzyl-1_H_-1,2,3-triazol-4-yl) methyl]amine. b, CAD mass spectrum of a tagged synthetic _O_-GlcNAc-modified peptide, YSPTgSPSK (gS is _O_-GlcNAcylated Ser), showing signature ions at m/z 300.2 and 503.1 that result from cleavage at the two sugar ketal linkages and that confirm the presence of the tagged _O_-GlcNAc moiety. c, ETD mass spectrum recorded on [M + 3H]3+ (m/z 457.2) ions from the same peptide, YSPTgSPSK. Predicted m/z values for ions of type c′ and z′· (monoisotopic and average masses for singly and doubly charged ions, respectively) are shown above and below the peptide sequence. Observed product ions are underlined and also labeled in the spectrum. Ions in the precursor isolation window are labeled with a triangle (▾). Brackets enclose ions that correspond to charge-reduced species and fragments derived from them by loss of small, neutral molecules. Product ions that result from loss of an aminomethyltriazole radical are labeled with a circle (○). M represents the tagged peptide.
Fig. 2.
Characterization of an _O_-GlcNAc-modified peptide from α-crystallin. a, CAD mass spectrum showing signature ions characteristic of the derivatized _O_-GlcNAc moiety. M represents the tagged peptide. b, ETD mass spectrum recorded on [M + 4H]4+ ions (m/z 537.10) from the same peptide, AIPVgSREEKPSSAPSS. Predicted m/z values for ions of type c′ and z′· (monoisotopic masses and average masses for singly and doubly charged ions, respectively) are shown above and below the peptide sequence. Observed product ions are underlined and also labeled in the spectrum. Ions in the precursor isolation window are labeled with a triangle (▾). Brackets enclose ions that correspond to charge-reduced species and fragments derived from them by loss of small, neutral molecules. Product ions that result from loss of an aminomethyltriazole radical are labeled with a circle (○). c, the tagging approach enables detection of _O_-GlcNAc-modified proteins by avidin-HRP blotting. This figure shows that tagged α-crystalline provides a strong signal after blotting with avidin-HRP blotting. Upon UV illumination, no signal is observed indicating photocleavage and loss of the biotin tag from α-crystallin.
Fig. 3.
Enriched and tagged _O_-GlcNAc-modified peptides from tau-containing protein fractions isolated from rat brain. a, ETD MS/MS spectrum recorded on [M + 3H]3+ ions (m/z 474.2) for the derivatized peptide VVgSDTSPR from the tau protein. b, ETD MS/MS spectrum recorded on [M + 3H]3+ ions (m/z 748.1) for the derivatized peptide VAAAAgTTTTTTTTTVAEK from MeCP2. Spectra are labeled as described in Figs. 1_c_ and 2_b_.
Similar articles
- O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry.
Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Maltby DA, Schoepfer R, Burlingame AL. Vosseller K, et al. Mol Cell Proteomics. 2006 May;5(5):923-34. doi: 10.1074/mcp.T500040-MCP200. Epub 2006 Feb 1. Mol Cell Proteomics. 2006. PMID: 16452088 - Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets.
Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG 2nd, Shabanowitz J, Stanley P, Hart GW, Hunt DF, Yang F, Smith RD. Alfaro JF, et al. Proc Natl Acad Sci U S A. 2012 May 8;109(19):7280-5. doi: 10.1073/pnas.1200425109. Epub 2012 Apr 19. Proc Natl Acad Sci U S A. 2012. PMID: 22517741 Free PMC article. - O-GlcNAc Site Mapping by Using a Combination of Chemoenzymatic Labeling, Copper-Free Click Chemistry, Reductive Cleavage, and Electron-Transfer Dissociation Mass Spectrometry.
Ma J, Wang WH, Li Z, Shabanowitz J, Hunt DF, Hart GW. Ma J, et al. Anal Chem. 2019 Feb 19;91(4):2620-2625. doi: 10.1021/acs.analchem.8b05688. Epub 2019 Feb 4. Anal Chem. 2019. PMID: 30657688 Free PMC article. - Methods for Enrichment and Assignment of N-Acetylglucosamine Modification Sites.
Maynard JC, Chalkley RJ. Maynard JC, et al. Mol Cell Proteomics. 2021;20:100031. doi: 10.1074/mcp.R120.002206. Epub 2021 Feb 9. Mol Cell Proteomics. 2021. PMID: 32938750 Free PMC article. Review. - The Utilities of Chemical Reactions and Molecular Tools for O-GlcNAc Proteomic Studies.
Kim EJ. Kim EJ. Chembiochem. 2015 Jul 6;16(10):1397-409. doi: 10.1002/cbic.201500183. Epub 2015 Jun 11. Chembiochem. 2015. PMID: 26096757 Review.
Cited by
- Click chemistry facilitates formation of reporter ions and simplified synthesis of amine-reactive multiplexed isobaric tags for protein quantification.
Sohn CH, Lee JE, Sweredoski MJ, Graham RL, Smith GT, Hess S, Czerwieniec G, Loo JA, Deshaies RJ, Beauchamp JL. Sohn CH, et al. J Am Chem Soc. 2012 Feb 8;134(5):2672-80. doi: 10.1021/ja2099003. Epub 2012 Jan 27. J Am Chem Soc. 2012. PMID: 22225568 Free PMC article. - Modulation of transcription factor function by O-GlcNAc modification.
Ozcan S, Andrali SS, Cantrell JE. Ozcan S, et al. Biochim Biophys Acta. 2010 May-Jun;1799(5-6):353-64. doi: 10.1016/j.bbagrm.2010.02.005. Epub 2010 Mar 2. Biochim Biophys Acta. 2010. PMID: 20202486 Free PMC article. Review. - Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis.
Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Wang Z, et al. Sci Signal. 2010 Jan 12;3(104):ra2. doi: 10.1126/scisignal.2000526. Sci Signal. 2010. PMID: 20068230 Free PMC article. - Targeting α-synuclein for PD Therapeutics: A Pursuit on All Fronts.
Teil M, Arotcarena ML, Faggiani E, Laferriere F, Bezard E, Dehay B. Teil M, et al. Biomolecules. 2020 Mar 3;10(3):391. doi: 10.3390/biom10030391. Biomolecules. 2020. PMID: 32138193 Free PMC article. Review. - Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles.
Iqbal K, Liu F, Gong CX. Iqbal K, et al. Biochem Pharmacol. 2014 Apr 15;88(4):631-9. doi: 10.1016/j.bcp.2014.01.002. Epub 2014 Jan 10. Biochem Pharmacol. 2014. PMID: 24418409 Free PMC article. Review.
References
- Hart G. W., Housley M. P., Slawson C. (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 - PubMed
- Kreppel L. K., Hart G. W. (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J. Biol. Chem 274, 32015–32022 - PubMed
- Wang Z., Hart G. W. (2008) Glycomic approaches to study GlcNAcylation: protein identification, site-mapping, and site-specific O-GlcNAc quantitation. Clin. Proteomics 4, 5–13
- Vosseller K., Trinidad J. C., Chalkley R. J., Specht C. G., Thalhammer A., Lynn A. J., Snedecor J. O., Guan S., Medzihradszky K. F., Maltby D. A., Schoepfer R., Burlingame A. L. (2006) O-Linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell. Proteomics 5, 923–934 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA42486/CA/NCI NIH HHS/United States
- R01 CA042486/CA/NCI NIH HHS/United States
- S10 RR023025/RR/NCRR NIH HHS/United States
- R01 GM037537/GM/NIGMS NIH HHS/United States
- R01 DK061671/DK/NIDDK NIH HHS/United States
- DK61671/DK/NIDDK NIH HHS/United States
- N01-HV-28180/HV/NHLBI NIH HHS/United States
- N01HV28180/HV/NHLBI NIH HHS/United States
- GM37537/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases