Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice - PubMed (original) (raw)
Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice
Tohru Kitada et al. J Neurochem. 2009 Nov.
Abstract
Recessively inherited loss-of-function mutations in the parkin, DJ-1, or PINK1 gene are linked to familial cases of early-onset Parkinson's diseases (PD), and heterozygous mutations are associated with increased incidence of late-onset PD. We previously reported that single knockout mice lacking Parkin, DJ-1, or PINK1 exhibited no nigral degeneration, even though evoked dopamine release from nigrostriatal terminals was reduced and striatal synaptic plasticity was impaired. In this study, we tested whether inactivation of all three recessive PD genes, each of which was required for nigral neuron survival in the aging human brain, resulted in nigral degeneration during the lifespan of mice. Surprisingly, we found that triple knockout mice lacking Parkin, DJ-1, and PINK1 have normal morphology and numbers of dopaminergic and noradrenergic neurons in the substantia nigra and locus coeruleus, respectively, at the ages of 3, 16, and 24 months. Interestingly, levels of striatal dopamine in triple knockout mice were normal at 16 months of age but increased at 24 months. These results demonstrate that inactivation of all three recessive PD genes is insufficient to cause significant nigral degeneration within the lifespan of mice, suggesting that these genes may be protective rather than essential for the survival of dopaminergic neurons during the aging process. These findings also support the notion that mammalian Parkin and PINK1 may function in the same genetic pathway as in Drosophila.
Figures
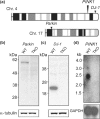
Fig. 1
(a) The mouse parkin gene is located on chromosome 17, whereas the mouse DJ-1 and PINK1 genes are both on chromosome 4, with an allelic distance of 12.5 cM. (b, c) Western blot analysis confirms the absence of Parkin (b) and DJ-1 (c) in TKO brains. The same blots were incubated with an anti-α-tubulin antibody as loading controls. (d) Northern analysis using a probe specific for exon 8 shows the absence of PINK1 transcripts in TKO brains. The same blot was hybridized with a GAPDH cDNA probe as control. WT, wild-type; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Fig. 2
No neurodegeneration in the SNpc and LC of TKO mice. (a–d) Normal immunoreactivity and morphology of SN neurons in TKO mice at the age of 24 months. Scale bars: 100 μm. (e) Similar numbers of TH-immunoreactive neurons are present in the SNpc of TKO mice and wild-type controls at the ages of 3 months (+/+: 12720 ± 992, n = 4; −/−: 11080 ± 740, n = 4; p > 0.05), 16 months (+/+: 11460 ± 1735, n = 4; −/−: 11880 ± 605, n = 4; p > 0.05), and 24 months (+/+: 11008 ± 736, n = 5; −/−: 10440 ± 396, n = 4; p > 0.05). All data are expressed as mean ± SEM. (f–i) Normal immunoreactivity and morphology of TH+ neurons in the LC of TKO mice at the age of 24 months. Scale bars: 100 μm. (j) Similar numbers of TH-immunoreactive neurons are present in the LC of TKO mice and wild-type controls at the ages of 3 months (+/+: 308 ± 16, n = 3; −/−: 298 ± 13, n = 4; p > 0.05), 16 months (+/+: 284 ± 38, n = 3; −/−: 236 ± 17, n = 4; p > 0.05), and 24 months (+/+: 231 ± 21, n = 4; −/−: 265 ± 16, n = 4; p > 0.05). All data are expressed as mean ± SEM.
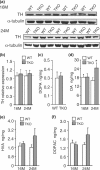
Fig. 3
Increased DA in the striatum of TKO mice at 24 months of age. (a and b) Western analysis showed similar levels of TH in TKO mice at the ages of 16 months (+/+: 1.00 ± 0.11, n = 4; −/−: 1.07 ± 0.15, n = 4; p > 0.05) and 24 months (+/+: 1.00 ± 0.19, n = 5; −/−: 1.08 ± 0.12, n = 5; p > 0.05). Each value of the TH protein level is normalized to that of α-tubulin. (c) TH activity assay. DOPA content in the TKO striatum following NSD-1015 treatment at the age of 2–5 months (0.33 ± 0.04 ng/mg, n = 4) was unchanged compared with wild-type controls (0.30 ± 0.07 ng/mg, n = 4; p > 0.05). Each data point represents the mean ± SEM. (d) Levels of striatal DA content are similar in TKO mice and wild-type controls at the age of 16 months (+/+: 18.4 ± 2.0 ng/mg, n = 5; −/−: 18.5 ± 2.3 ng/mg, n = 5; p > 0.05) and increased in TKO mice at 24 months (+/+: 16.3 ± 1.8 ng/mg, n = 5; −/−: 24.6 ± 3.4 ng/mg, n = 4; *p < 0.05). (e) Levels of striatal HVA in TKO mice and wild-type controls at the ages of 16 months (+/+: 1.76 ± 0.28 ng/mg, _n_ = 5; −/−: 1.98 ± 0.18 ng/mg, _n_ = 5; _p_ > 0.05) and 24 months (+/+: 1.65 ± 0.23 ng/mg, n = 5; −/−: 2.67 ± 0.65 ng/mg, n = 4; p > 0.05). (f) Levels of striatal DOPAC in TKO mice and wild-type controls at the ages of 16 months (+/+: 1.53 ± 0.20 ng/mg, n = 5; −/−: 1.42 ± 0.18 ng/mg, n = 5; p > 0.05) and 24 months (+/+: 1.42 ± 0.13 ng/mg, n = 5; −/−: 2.09 ± 0.46 ng/mg, n = 4; p > 0.05). All data are expressed as mean ± SEM.
Similar articles
- Behavioral and neurotransmitter abnormalities in mice deficient for Parkin, DJ-1 and superoxide dismutase.
Hennis MR, Seamans KW, Marvin MA, Casey BH, Goldberg MS. Hennis MR, et al. PLoS One. 2013 Dec 26;8(12):e84894. doi: 10.1371/journal.pone.0084894. eCollection 2013. PLoS One. 2013. PMID: 24386432 Free PMC article. - Unaltered striatal dopamine release levels in young Parkin knockout, Pink1 knockout, DJ-1 knockout and LRRK2 R1441G transgenic mice.
Sanchez G, Varaschin RK, Büeler H, Marcogliese PC, Park DS, Trudeau LE. Sanchez G, et al. PLoS One. 2014 Apr 14;9(4):e94826. doi: 10.1371/journal.pone.0094826. eCollection 2014. PLoS One. 2014. PMID: 24733019 Free PMC article. - Surprising behavioral and neurochemical enhancements in mice with combined mutations linked to Parkinson's disease.
Hennis MR, Marvin MA, Taylor CM 2nd, Goldberg MS. Hennis MR, et al. Neurobiol Dis. 2014 Feb;62:113-23. doi: 10.1016/j.nbd.2013.09.009. Epub 2013 Sep 26. Neurobiol Dis. 2014. PMID: 24075852 Free PMC article. - Evidence for a common biological pathway linking three Parkinson's disease-causing genes: parkin, PINK1 and DJ-1.
van der Merwe C, Jalali Sefid Dashti Z, Christoffels A, Loos B, Bardien S. van der Merwe C, et al. Eur J Neurosci. 2015 May;41(9):1113-25. doi: 10.1111/ejn.12872. Epub 2015 Mar 11. Eur J Neurosci. 2015. PMID: 25761903 Review. - Recessive Parkinson's disease.
Kubo S, Hattori N, Mizuno Y. Kubo S, et al. Mov Disord. 2006 Jul;21(7):885-93. doi: 10.1002/mds.20841. Mov Disord. 2006. PMID: 16615060 Review.
Cited by
- Early exposure to paraquat sensitizes dopaminergic neurons to subsequent silencing of PINK1 gene expression in mice.
Zhou H, Huang C, Tong J, Xia XG. Zhou H, et al. Int J Biol Sci. 2011;7(8):1180-7. doi: 10.7150/ijbs.7.1180. Epub 2011 Oct 26. Int J Biol Sci. 2011. PMID: 22043175 Free PMC article. - Epigallocatechin-3-Gallate Protects and Prevents Paraquat-Induced Oxidative Stress and Neurodegeneration in Knockdown dj-1-β Drosophila melanogaster.
Martinez-Perez DA, Jimenez-Del-Rio M, Velez-Pardo C. Martinez-Perez DA, et al. Neurotox Res. 2018 Oct;34(3):401-416. doi: 10.1007/s12640-018-9899-x. Epub 2018 Apr 17. Neurotox Res. 2018. PMID: 29667128 - BAG2 Gene-mediated Regulation of PINK1 Protein Is Critical for Mitochondrial Translocation of PARKIN and Neuronal Survival.
Qu D, Hage A, Don-Carolis K, Huang E, Joselin A, Safarpour F, Marcogliese PC, Rousseaux MW, Hewitt SJ, Huang T, Im DS, Callaghan S, Dewar-Darch D, Figeys D, Slack RS, Park DS. Qu D, et al. J Biol Chem. 2015 Dec 18;290(51):30441-52. doi: 10.1074/jbc.M115.677815. Epub 2015 Nov 4. J Biol Chem. 2015. PMID: 26538564 Free PMC article. - Molecular, Neurochemical, and Behavioral Hallmarks of Reserpine as a Model for Parkinson's Disease: New Perspectives to a Long-Standing Model.
Leão AH, Sarmento-Silva AJ, Santos JR, Ribeiro AM, Silva RH. Leão AH, et al. Brain Pathol. 2015 Jul;25(4):377-90. doi: 10.1111/bpa.12253. Epub 2015 Apr 22. Brain Pathol. 2015. PMID: 25726735 Free PMC article. Review. - Il-10 signaling reduces survival in mouse models of synucleinopathy.
Cockey SG, McFarland KN, Koller EJ, Brooks MMT, Gonzalez De La Cruz E, Cruz PE, Ceballos-Diaz C, Rosario AM, Levites YR, Borchelt DR, Golde TE, Giasson BI, Chakrabarty P. Cockey SG, et al. NPJ Parkinsons Dis. 2021 Mar 19;7(1):30. doi: 10.1038/s41531-021-00169-8. NPJ Parkinsons Dis. 2021. PMID: 33741985 Free PMC article.
References
- Albanese A, Valente EM, Romito LM, Bellacchio E, Elia AE, Dallapiccola B. The PINK1 phenotype can be indistinguishable from idiopathic Parkinson disease. Neurology. 2005;64:1958–1960. - PubMed
- Arrue A, Ruiz-Ortega JA, Ugedo L, Giralt MT. Short-term effects of 3,4-methylenedioximethamphetamine on noradrenergic activity in locus coeruleus and hippocampus of the rat. Neurosci. Lett. 2003;337:123–126. - PubMed
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann. N Y Acad. Sci. 2003;991:120–131. - PubMed
- Bonifati V, Breedveld GJ, Squitieri F, et al. Localization of autosomal recessive early-onset parkinsonism to chromosome 1p36 (PARK7) in an independent dataset. Ann. Neurol. 2002;51:253–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources