A non-redundant role for MKP5 in limiting ROS production and preventing LPS-induced vascular injury - PubMed (original) (raw)
A non-redundant role for MKP5 in limiting ROS production and preventing LPS-induced vascular injury
Feng Qian et al. EMBO J. 2009.
Abstract
There are at least 11 mitogen-activated protein kinase (MAPK) phosphatases (MKPs) and only 3 major groups of MAPKs, raising the question of whether these phosphatases have non-redundant functions in vivo. Using a modified mouse model of local Shwartzman reaction, we found that deletion of the MKP5 gene, but not the MKP1 gene, led to robust and accelerated vascular inflammatory responses to a single dose of LPS injection. Depletion of neutrophils significantly reduced the vascular injury in Mkp5(-/-) mice, whereas adoptive transfer of Mkp5(-/-) neutrophils replicated the LPS-induced skin lesions in wild-type recipients. Neutrophils isolated from Mkp5(-/-) mice exhibited augmented p38 MAPK activation and increased superoxide generation on activation. The p38 MAPK inhibitor, SB203580, significantly reduced p47(phox) phosphorylation and diminished superoxide production in neutrophils. p38 MAPK phosphorylated mouse p47(phox), and deletion of the p47(phox) gene ablated the LPS-induced vascular injury in Mkp5(-/-) mice. Collectively, these results show an earlier unrecognized and non-redundant function of MKP5 in restraining p38 MAPK-mediated neutrophil oxidant production, thereby preventing LPS-induced vascular injury.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
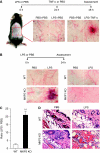
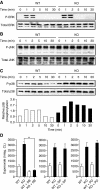
LPS-induced microvascular injury in Mkp5+/+ and _Mkp5_−/− mice. (A) Experimental scheme and results of a classical local Shwartzman reaction (LSR) induced by consecutive injections of LPS and then TNF-α. Dorsal skin of WT mice were first injected s.c. with LPS (80 μg, right side of each panel) or PBS control (left side). After 24 h, TNF-α (0.2 μg) or PBS in same volume was injected s.c. into the same site that received LPS. The mice were killed 24 h after the second injection, and the skin tissues were examined macroscopically. Representative sample images from one of the five experiments are shown. (B) Experimental scheme and results of a modified (one-injection) LSR showing macroscopic appearance of dorsal skin in Mkp5+/+ (WT) and _Mkp5_−/− (KO) mice (panels on the left), compared with that of the Mkp1+/+ (WT) and _Mkp1_−/− (KO) mice (panels on the right). The WT and KO littermates in each group received an injection of either LPS (80 μg; right to the dotted line) or PBS (left to the dotted line). A total of 11 WT and 15 _Mkp5_−/− mice, and 8 WT and 8 _Mkp1_−/− mice were examined 24 h after the LPS injection. Representative images are shown. (C) The degree of haemorrhage in the Mkp5+/+ and _Mkp5_−/− group above was estimated based on densitometry analysis of skin samples receiving either LPS or PBS injection. **P<0.01. (D) Grouped images showing representative photomicrographs of H&E-stained skin sections from WT (upper panels) and _Mkp5_−/− (lower panels) mice that were treated with LPS or PBS as marked. Erythrocyte extravasation, thrombus formation and neutrophil accumulation are evident in the sample from LPS-treated _Mkp5_−/− mice 24 h after LPS injection.
Figure 2
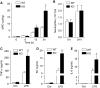
LPS-induced tissue neutrophilia and cytokine production in Mkp5+/+ and _Mkp5_−/− mice. (A) Time-dependent neutrophil accumulation at the LPS injection sites in WT and _Mkp5_−/− (KO) mice, based on the activity of neutrophil myeloperoxidase (MPO) in tissue extract. The values of MPO activity represent the means±s.e.m. of measurements in four mice. The difference between the WT and KO mice at 12 and 24 h time points was statistically significant (*P<0.05). (B) Peripheral blood neutrophil count in WT and _Mkp5_−/− (KO) mice before (0 h) and after (24 h) receiving 80 μg LPS (_n_=5). (C–E) Change in the expression level of TNF-α, KC and IL-6 in WT and _Mkp5_−/− mice. Air pouch was established by injecting sterile air into the dorsal skin of WT and _Mkp5_−/− (KO) mice. LPS (80 μg) was then injected into the air pouch. After 2 h, lavage fluid was collected from the air pouch and the concentration of TNF-α, KC and IL-6 was determined. Values shown are means±s.e.m. of measurements using four mice. *P<0.05 and **P<0.01, compared with LPS-stimulated WT mice.
Figure 3
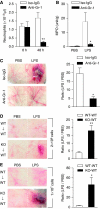
Role of neutrophils in LPS-induced microvascular injury in _Mkp5_−/− mice. (A) Peripheral blood neutrophil count showing anti-Gr-1-mediated depletion of neutrophils in _Mkp5_−/− mice, determined at 0 and 48 h after i.p. injection of the antibody (filled bars) and compared with mice receiving isotype-matched IgG (Iso-IgG, open bars). (B) Neutrophil accumulation at the site of LPS or PBS injection in _Mkp5_−/− mice receiving anti-Gr-1 (filled bars) or isotype-matched IgG (open bars). (C) Macroscopic appearance of dorsal skin in LPS-injected _Mkp5_−/− mice that received anti-Gr-1 or isotype-matched IgG (100 μg each) 24 h before LPS challenge. Representative images from five independent experiments are shown. The degree of haemorrhage was quantified by densitometry and shown on the right side. (D, E) WT mice were intravenously injected with bone marrow neutrophils (2 × 106 in D, and 1 × 107 in E) isolated from either _Mkp5_−/− mice (KO to WT) or WT mice (WT to WT). After 10 min, dorsal skin was injected s.c. with LPS (80 μg). Macroscopic appearance of the LPS injection site was imaged at 24 h (_n_=5 in each group), and representative images are shown. For (C), (D) and (E), the degree of haemorrhage was estimated by densitometry as described in Figure 1, and the results are shown as means±s.e.m. on the right side of the images. *P<0.05; **P<0.01, based on five mice in each group.
Figure 4
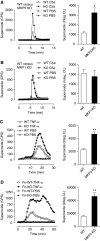
Augmented superoxide production in _Mkp5_−/− neutrophils. Representative tracings showing the production of superoxide as counts per second (CPS) by neutrophils (5 × 105) from the _Mkp5_−/− mice (A), _Mkp1_−/− mice (B) and their WT littermates, stimulated in suspension with 100 nM C5a or PBS. In (C) neutrophils from the _Mkp5_−/− mice and their WT littermates were stimulated with 100 ng/ml TNF-α or PBS, and superoxide production was measured. In (D) neutrophils from _Mkp5_−/− mice and their WT littermates were let adherent to fibrinogen-coated surface and stimulated with 100 ng/ml TNF-α. Superoxide generation in these experiments was detected using isoluminol-ECL. Shown on the right to the tracings are quantifications of cumulative superoxide production as means±s.e.m., based on measurement of integrated chemiluminescence (CL) in the course of the assays as indicated (_n_=5). *P<0.05; **P<0.01.
Figure 5
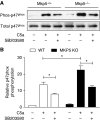
C5a-induced MAPK activation and superoxide production in WT and _Mkp5_−/− neutrophils. Phosphorylation of ERK1/2 (A), JNK (B) and p38 MAPK (C) in C5a (10 nM)-stimulated WT and _Mkp5_−/− neutrophils was determined by western blotting using anti-phospho-antibodies against the individual MAPKs. Total MAPKs in the samples were shown below the phospho-MAPK blots. Densitometry analysis was conducted to determine the relative level of induced p38 MAPK phosphorylation, and the results are shown in (C). (D) The effects of MAPK inhibitors on C5a-stimulated superoxide production in WT (open bars) and _Mkp5_−/− (KO, filled bars) neutrophils, shown as integrated chemiluminescence (CL) in a 15 min period after stimulation. The cells were treated for 15 min with either the p38 MAPK inhibitor SB203580 (SB, 3 μM), the JNK inhibitor SP600125 (SP, 10 μM) or the MEK inhibitor PD98059 (PD, 30 μM), before C5a stimulation. Data shown are means±s.e.m. from three independent experiments. **P<0.01.
Figure 6
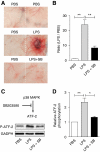
In vivo effects of SB203580 on LPS-induced vascular injury and p38 MAPK activation. The p38 MAPK inhibitor SB203580 (100 μg) was co-administered s.c. with LPS (80 μg) to _Mkp5_−/− mice. Control mice received either PBS or LPS alone. The mice were killed 24 h after injection, and skin tissue at the injection site was examined (A). The degree of a haemorrhage was quantified by densitometry analysis of skin sample and shown in (B). (C) Skin tissues at the injection site were excised and ATF-2 phosphorylation in tissue homogenate was determined by western blotting, using an antibody recognizing phosphorylated ATF-2. The relative level of ATF-2 phosphorylation was shown in (D). **P<0.01 and *P<0.05, based on four experiments.
Figure 7
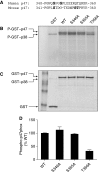
Identification of Thr356 in mouse p47phox as a p38 MAPK phosphorylation site. (A) Alignment of sequence of human and mouse p47phox proteins surrounding the potential p38 MAPK phosphorylation site. (B) Autoradiograph of in vitro kinase assay using full-length WT or mutated mp47phox fused to GST as substrates and an activated GST–p38α, as detailed in the section ‘Materials and methods'. GST without mp47phox was used as a negative control. Two phosphorylated bands were identified in the autoradiograph: a phosphorylated GST–mp47phox (apparent molecular weight 72 kDa), and an autophosphorylated GST–p38 (apparent molecular weight 68 kDa). (C) Coomassie blue staining showing the positions and levels of the proteins in the gel. (D) The extent of substrate phosphorylation was quantified by densitometry, and fold change relative to the phosphorylated WT GST–mp47phox was shown. Two independent kinase assays were performed, and similar results were obtained.
Figure 8
Posphorylation of p47phox in WT and _Mkp5_−/− neutrophils on C5a stimulation. Neutrophils from _Mkp5_−/− and WT littermates were pre-treated with SB203580 (3 μM) or vehicle for 15 min, and then stimulated with C5a (100 nM) for 1 min. The phosphorylated proteins from neutrophil lysate were collected through affinity chromatography as described in the section ‘Materials and methods'. (A) Western blots showing phosphorylated p47phox (in the eluate) and total p47phox (in the cell lysate), detected with an anti-p47phox antibody. (B) Densitometry analysis was conducted to determine the relative level of mouse p47phox phosphorylation based on three independent experiments. *P<0.05.
Figure 9
Superoxide production and vascular response in p47 _phox_−/− _Mkp5_−/− mice. (A) and (B) Absence of superoxide production by p47 _phox_−/− neutrophils. Bone marrow neutrophils from p47 phox+/+ _Mkp5_−/− and p47 _phox_−/− _Mkp5_−/− mice (5 × 105 cells/sample) were stimulated with 100 nM C5a (A) or 200 ng/ml PMA (B), using PBS as a negative control. Superoxide production was shown as counts per second (CPS) in isoluminol-ECL. A representative set of tracings from five similar experiments is shown. (C) Representative images showing macroscopic appearance of the LPS injection sites in p47 phox+/+ _Mkp5_−/− mice (_n_=8) and p47 _phox_−/− _Mkp5_−/− mice (_n_=7), observed 24 h after s.c. injection with LPS (80 μg) or PBS. The degree of haemorrhage in LPS-injected mice was estimated as described in Figure 1. (D) Representative photomicrographs of H&E-stained skin sections from p47 phox+/+ (upper panels) and p47 _phox_−/− (lower panels) _Mkp5_−/− mice that received dermal injection with LPS (right side) or PBS (left side). Erythrocyte extravasation and occlusive thrombi containing neutrophils were visible in the sample from p47 phox+/+ _Mkp5_−/− mice but not p47 _phox_−/− _Mkp5_−/− mice. Magnification= × 400. **P<0.01.
Similar articles
- Map kinase phosphatase 5 protects against sepsis-induced acute lung injury.
Qian F, Deng J, Gantner BN, Flavell RA, Dong C, Christman JW, Ye RD. Qian F, et al. Am J Physiol Lung Cell Mol Physiol. 2012 May 1;302(9):L866-74. doi: 10.1152/ajplung.00277.2011. Epub 2012 Feb 3. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22307906 Free PMC article. - Ketamine reduces inducible superoxide generation in human neutrophils in vitro by modulating the p38 mitogen-activated protein kinase (MAPK)-mediated pathway.
Lu HW, He GN, Ma H, Wang JK. Lu HW, et al. Clin Exp Immunol. 2010 Jun;160(3):450-6. doi: 10.1111/j.1365-2249.2010.04111.x. Epub 2010 Mar 16. Clin Exp Immunol. 2010. PMID: 20345980 Free PMC article. - p38 Mitogen-activated protein kinase and extracellular signal-regulated kinase signaling pathways are not essential regulators of formyl peptide-stimulated p47(phox) activation in neutrophils.
Tsai YR, Wang YJ, Lee MR, Hsu MF, Wang JP. Tsai YR, et al. Eur J Pharmacol. 2013 Feb 15;701(1-3):96-105. doi: 10.1016/j.ejphar.2013.01.003. Epub 2013 Jan 21. Eur J Pharmacol. 2013. PMID: 23348708 - Nepetoidin B Alleviates Liver Ischemia/Reperfusion Injury via Regulating MKP5 and JNK/P38 Pathway.
Yu Q, Mei C, Cui M, He Q, Liu X, Du X. Yu Q, et al. Drug Des Devel Ther. 2024 Jun 17;18:2301-2315. doi: 10.2147/DDDT.S457130. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38911032 Free PMC article. - Review of Defective NADPH Oxidase Activity and Myeloperoxidase Release in Neutrophils From Patients With Cirrhosis.
Moreau R, Périanin A, Arroyo V. Moreau R, et al. Front Immunol. 2019 May 8;10:1044. doi: 10.3389/fimmu.2019.01044. eCollection 2019. Front Immunol. 2019. PMID: 31134093 Free PMC article. Review.
Cited by
- Dual-specificity MAP kinase phosphatases in health and disease.
Seternes OM, Kidger AM, Keyse SM. Seternes OM, et al. Biochim Biophys Acta Mol Cell Res. 2019 Jan;1866(1):124-143. doi: 10.1016/j.bbamcr.2018.09.002. Epub 2018 Sep 8. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30401534 Free PMC article. Review. - The Prolyl Isomerase Pin1 Controls Lipopolysaccharide-Induced Priming of NADPH Oxidase in Human Neutrophils.
Liu M, Bedouhene S, Hurtado-Nedelec M, Pintard C, Dang PM, Yu S, El-Benna J. Liu M, et al. Front Immunol. 2019 Nov 1;10:2567. doi: 10.3389/fimmu.2019.02567. eCollection 2019. Front Immunol. 2019. PMID: 31736979 Free PMC article. - A Role for MK2 in Enhancing Neutrophil-Derived ROS Production and Aggravating Liver Ischemia/Reperfusion Injury.
Sun L, Wu Q, Nie Y, Cheng N, Wang R, Wang G, Zhang D, He H, Ye RD, Qian F. Sun L, et al. Front Immunol. 2018 Nov 13;9:2610. doi: 10.3389/fimmu.2018.02610. eCollection 2018. Front Immunol. 2018. PMID: 30483268 Free PMC article. - IκB kinase-beta inhibitor attenuates hepatic fibrosis in mice.
Wei J, Shi M, Wu WQ, Xu H, Wang T, Wang N, Ma JL, Wang YG. Wei J, et al. World J Gastroenterol. 2011 Dec 21;17(47):5203-13. doi: 10.3748/wjg.v17.i47.5203. World J Gastroenterol. 2011. PMID: 22215946 Free PMC article. - Moesin and myosin phosphatase confine neutrophil orientation in a chemotactic gradient.
Liu X, Yang T, Suzuki K, Tsukita S, Ishii M, Zhou S, Wang G, Cao L, Qian F, Taylor S, Oh MJ, Levitan I, Ye RD, Carnegie GK, Zhao Y, Malik AB, Xu J. Liu X, et al. J Exp Med. 2015 Feb 9;212(2):267-80. doi: 10.1084/jem.20140508. Epub 2015 Jan 19. J Exp Med. 2015. PMID: 25601651 Free PMC article.
References
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P (1995) Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol 5: 283–295 - PubMed
- Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342–344 - PubMed
- Bhalla US, Ram PT, Iyengar R (2002) MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 297: 1018–1023 - PubMed
- Brozna JP (1990) Shwartzman reaction. Semin Thromb Hemost 16: 326–332 - PubMed
- Camps M, Nichols A, Arkinstall S (2000) Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14: 6–16 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous