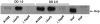Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains - PubMed (original) (raw)
Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains
Takahiko Ishikawa et al. PLoS One. 2009.
Abstract
Background: The type VI secretion system (T6SS) has emerged as a protein secretion system important to several gram-negative bacterial species. One of the common components of the system is Hcp, initially described as a hemolysin co-regulated protein in a serotype O17 strain of Vibrio cholerae. Homologs to V. cholerae hcp genes have been found in all characterized type VI secretion systems and they are present also in the serotype O1 strains of V. cholerae that are the cause of cholera diseases but seemed to have non-functional T6SS.
Methodology/principal findings: The serotype O1 V. cholerae strain A1552 was shown to express detectable levels of Hcp as determined by immunoblot analyses using polyclonal anti-Hcp antiserum. We found that the expression of Hcp was growth phase dependent. The levels of Hcp in quorum sensing deficient mutants of V. cholerae were compared with the levels in wild type V. cholerae O1 strain A1552. The expression of Hcp was positively and negatively regulated by the quorum sensing regulators HapR and LuxO, respectively. In addition, we observed that expression of Hcp was dependent on the cAMP-CRP global transcriptional regulatory complex and required the RpoN sigma factor.
Conclusion/significance: Our results show that serotype O1 strains of V. cholerae do express Hcp which is regarded as one of the important T6SS components and is one of the secreted substrates in non-O1 non-O139 V. cholerae isolates. We found that expression of Hcp was strictly regulated by the quorum sensing system in the V. cholerae O1 strain. In addition, the expression of Hcp required the alternative sigma factor RpoN and the cAMP-CRP global regulatory complex. Interestingly, the environmental isolates of V. cholerae O1 strains that showed higher levels of the HapR quorum sensing regulator in comparison with our laboratory standard serotype O1 strain A1552 where also expressing higher levels of Hcp.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Hcp levels in V. cholerae O1 wild type strain A1552 at different growth phases.
Bacteria were grown as described in Materials and Methods and the whole cell lysate samples were taken at different optical density. Immunoblot analysis was performed with an anti-Hcp antiserum that also contained antibodies recognizing the OmpA major outer membrane protein as confirmed by analysis of ompA mutant V. cholerae (data not shown).
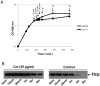
Figure 2. Analysis of Hcp stability in V. cholerae O1 wild type strain A1552.
(A) The growth curve and time of sampling for Hcp analyses of V. cholerae wild type strain A1552 with and without chloramphenicol (Cm) treatment. The bacterial cells were grown to OD 2.0 and 25 µg/ml Cm was added. Arrows indicate the time points of sampling for immunoblot analysis after the addition of Cm. (B) Immunoblot analysis of the stability of Hcp in V. cholerae wild type strain A1552. The samples were taken at different time points after the addition of Cm (for the test sample). For the control experiment, the samples were taken at different time points during normal growth of bacteria. The vertical arrows show the time points when the samples were taken.
Figure 3. The effect of Δ_hapR_ and Δ_luxO_ mutations on Hcp levels in V. cholerae strain A1552.
The whole cell lysate samples were taken at OD 1.0 and OD 2.0 and immunoblot analysis was performed using anti-Hcp polyclonal antiserum.
Figure 4. Hcp levels in different quorum sensing regulatory system of V. cholerae strain A1552.
The samples were taken at OD 1.0 and OD 2.0 from wild type V. cholerae O1 strain A1552 and its quorum sensing regulator mutants and immunoblot analyses were done using anti-Hcp antiserum
Figure 5. Hcp levels in Δ_crp_ and Δ_cya_ mutants of V. cholerae strain 1552.
Immunoblot analysis of Hcp levels in whole cell lysates of V. cholerae O1 wild type strain A1552, Δ_crp_, Δ_cya_, Δ_crp_/p_crp_, and Δ_crp_/vector control strains. The whole cell lysates were taken at OD 1.0 and OD 2.0 and immunoblot was done using anti-Hcp antiserum.
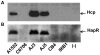
Figure 6. Levels of Hcp and HapR in different V. cholerae O1 isolates.
(A) Immunoblot analysis of the expression of the Hcp in different V. cholerae O1 isolates was performed using anti-Hcp antiserum. (B) Detection of the HapR protein in same samples used for the detection of the Hcp. Samples were taken at OD 2.0. (-); negative controls for Hcp (Δ_hcp_1,2) and HapR (Δ_hapR_)
Figure 7. The _hcp_1 and _hcp_2 loci in V. cholerae strain A1552 and levels of global regulators.
(A) DNA sequences of the promoter regions and part of open reading frames of _hcp_1 (a) and _hcp_2 (b) genes in V. cholerae O1 strain A1552. A potential ribosome binding sites (RBS), IHF binding sites (IHF), and sigma 54 binding sites (σ54) are underlined. The position corresponding to reported transcriptional start points in a serotype O17 V. cholerae strain are labeled as +1. The amino acid sequences of the ORFs are also shown above the nucleotide sequences in bold type. (B) The levels of Hcp, CRP, HapR, and RpoN in the V. cholerae O1 wild type strain. Immunoblot analyses of whole cell extracts from V. cholerae O1 wild type strain A1552 at OD 1.0 and 2.0 was performed using anti-Hcp, anti-CRP, and anti-HapR polyclonal antisera.
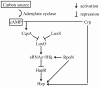
Figure 8. Regulation of Hcp expression in the V. cholerae O1 strain A1552.
A schematic summary of the involvement of different regulators in the growth phase dependent expression of Hcp in strain A1552.
Similar articles
- Expression, secretion and bactericidal activity of type VI secretion system in Vibrio anguillarum.
Tang L, Yue S, Li GY, Li J, Wang XR, Li SF, Mo ZL. Tang L, et al. Arch Microbiol. 2016 Oct;198(8):751-60. doi: 10.1007/s00203-016-1236-2. Epub 2016 May 12. Arch Microbiol. 2016. PMID: 27172981 - Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae.
Zheng J, Shin OS, Cameron DE, Mekalanos JJ. Zheng J, et al. Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21128-33. doi: 10.1073/pnas.1014998107. Epub 2010 Nov 17. Proc Natl Acad Sci U S A. 2010. PMID: 21084635 Free PMC article. - Pathoadaptive conditional regulation of the type VI secretion system in Vibrio cholerae O1 strains.
Ishikawa T, Sabharwal D, Bröms J, Milton DL, Sjöstedt A, Uhlin BE, Wai SN. Ishikawa T, et al. Infect Immun. 2012 Feb;80(2):575-84. doi: 10.1128/IAI.05510-11. Epub 2011 Nov 14. Infect Immun. 2012. PMID: 22083711 Free PMC article. - Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios.
Ball AS, Chaparian RR, van Kessel JC. Ball AS, et al. J Bacteriol. 2017 Sep 5;199(19):e00105-17. doi: 10.1128/JB.00105-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28484045 Free PMC article. Review. - Epidemiology & molecular biology of Vibrio cholerae O139 Bengal.
Albert MJ. Albert MJ. Indian J Med Res. 1996 Jul;104:14-27. Indian J Med Res. 1996. PMID: 8783504 Review.
Cited by
- Relative contributions of Vibrio polysaccharide and quorum sensing to the resistance of Vibrio cholerae to predation by heterotrophic protists.
Sun S, Kjelleberg S, McDougald D. Sun S, et al. PLoS One. 2013;8(2):e56338. doi: 10.1371/journal.pone.0056338. Epub 2013 Feb 18. PLoS One. 2013. PMID: 23441178 Free PMC article. - Comparative secretomics reveals novel virulence-associated factors of Vibrio parahaemolyticus.
He Y, Wang H, Chen L. He Y, et al. Front Microbiol. 2015 Jul 17;6:707. doi: 10.3389/fmicb.2015.00707. eCollection 2015. Front Microbiol. 2015. PMID: 26236293 Free PMC article. - A multidrug resistance plasmid contains the molecular switch for type VI secretion in Acinetobacter baumannii.
Weber BS, Ly PM, Irwin JN, Pukatzki S, Feldman MF. Weber BS, et al. Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9442-7. doi: 10.1073/pnas.1502966112. Epub 2015 Jul 13. Proc Natl Acad Sci U S A. 2015. PMID: 26170289 Free PMC article. - Quorum sensing-regulated chitin metabolism provides grazing resistance to Vibrio cholerae biofilms.
Sun S, Tay QX, Kjelleberg S, Rice SA, McDougald D. Sun S, et al. ISME J. 2015 Aug;9(8):1812-20. doi: 10.1038/ismej.2014.265. Epub 2015 Jan 23. ISME J. 2015. PMID: 25615438 Free PMC article. - Response of Vibrio cholerae to Low-Temperature Shifts: CspV Regulation of Type VI Secretion, Biofilm Formation, and Association with Zooplankton.
Townsley L, Sison Mangus MP, Mehic S, Yildiz FH. Townsley L, et al. Appl Environ Microbiol. 2016 Jun 30;82(14):4441-52. doi: 10.1128/AEM.00807-16. Print 2016 Jul 15. Appl Environ Microbiol. 2016. PMID: 27208110 Free PMC article.
References
- Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner's guide. Curr Opin Microbiol. 2008;11:3–8. - PubMed
- Rao PS, Yamada Y, Tan YP, Leung KY. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol Microbiol. 2004;53:573–586. - PubMed
- Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66:1192–1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous