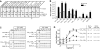Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion - PubMed (original) (raw)
Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion
Enfu Hui et al. Cell. 2009.
Abstract
Decades ago it was proposed that exocytosis involves invagination of the target membrane, resulting in a highly localized site of contact between the bilayers destined to fuse. The vesicle protein synaptotagmin-I (syt) bends membranes in response to Ca(2+), but whether this drives localized invagination of the target membrane to accelerate fusion has not been determined. Previous studies relied on reconstituted vesicles that were already highly curved and used mutations in syt that were not selective for membrane-bending activity. Here, we directly address this question by utilizing vesicles with different degrees of curvature. A tubulation-defective syt mutant was able to promote fusion between highly curved SNARE-bearing liposomes but exhibited a marked loss of activity when the membranes were relatively flat. Moreover, bending of flat membranes by adding an N-BAR domain rescued the function of the tubulation-deficient syt mutant. Hence, syt-mediated membrane bending is a critical step in membrane fusion.
Figures
Figure 1. Biophysical analyses of membrane curvature sensing ability of syt
(A) Model depicting the interaction of the cytoplasmic domain of syt (C2A-C2B) with membranes. The solution structure of C2A and C2B were rendered from (Shao et al., 1998) and (Fernandez et al., 2001). Ca2+ ions = red spheres. Two Ca2+ binding loops (L1 and L3) in each C2 domain partially penetrate the lipid bilayer in response to Ca2+. (B) Co-sedimentation experiments were used to assess the membrane-curvature preference of C2A-C2B. Shown is a representative gel of the remaining protein in the supernatant of each sample. With increasing [lipid], C2A-C2B was depleted from the supernatant fraction. (C) Quantification of the gels in panel B; error bars represent SEM from triplicate determinations. (D) Representative calorimetric heat flow signals obtained when vesicles are injected into a solution containing C2A-C2B. V105nm suspension (20 mM [lipid]) was titrated against C2A-C2B (20 μM) in the presence of 1 mM Ca2+ (top panel), 0.2 mM EGTA (middle panel), or V252nm in 1 mM Ca2+ (lower panel). (E) Integrated heat plotted as a function of [lipid]/[C2A-C2B]; thermodynamic parameters are summarized in Table S2. The binding isotherm of V252nm is right-shifted as compared to that of V105nm. (F) Reaction scheme for the stopped-flow rapid mixing experiments; vesicle = blue sphere. (G) Representative traces of the time course of Ca2+•C2A-C2B assembly with V105nm and V252nm, at 1 mM [lipid]. (H) _k_obs was plotted as a function of [lipid]. Kinetic parameters are summarized in Table S3. (I) Fold-increase in C2A-C2B•membrane complex _K_d values as a function of liposome diameter.
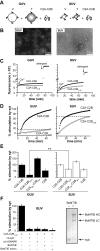
Figure 2. Syt utilizes C2B to bend membranes in a response to Ca2+
(A-L) Electron micrographs of Folch liposomes incubated with the indicated syt fragments (10 μM) for 10 hr. Scale bars correspond to 200 nm. The diameters of the lipid tubules were (mean ± SD, n = 30): 16 ± 4 nm for C2A-C2B; 11 ± 2 nm for C2B and 10 ± 3 nm for C2A3W-C2B3W. (M) Model showing that different degrees of membrane-bending may result in different morphologies of membranes. Membranes are shown in gray, syt fragments are shown as small ovals. Positive and negative curvatures are indicated as ‘+’ and ‘−’, respectively. Penetration of syt into the membrane drives deformation of the bilayer. The resulting morphologies can be classified into four categories, as follows: (M1), formation of a ‘nipple’, which has positive curvature at the tip, but negative curvature at the base, as indicated by dashed arrows. Protein prefers positively curved membranes and tends to cluster at the nipple tip. This in turn helps to maintain the shape of the nipple. (M2), more extensive membrane bending causes elongation of the nipple, forming a lipid tubule protruding from the parental liposome. New areas with a high degree of positive curvature emerge on the radial plane resulting in increased membrane area that is favorable for protein binding. (M3), even more extensive membrane bending would transform a whole liposome into a long tubule, removing any negative curvature. (M4), in extreme cases, the long lipid tubules are broken into short tubules or even tiny vesicles, further increasing the area with positive curvature. (N) Quantification of the fraction of tubulated liposomes obtained in the presence of the indicated syt construct.
Figure 3. Mutations that alter membrane-bending also affect the ability of syt to bind t-SNAREs
(A) Co-immunoprecipitation (IP) of syt fragments with t-SNARE heterodimers was carried out using an anti-syntaxin (syx) antibody. Equal fractions of total input (T) and immunoprecipitated material (E: 0.2 mM EGTA; Ca: 1 mM Ca2+) were subjected to SDS-PAGE. The 31 samples were resolved in two separate gels, which were juxtaposed at the position indicated by the arrow. (B) t-SNARE binding activity of each syt protein was quantified by densitometry. Data are reported as mean ± SD, n = 3. (C) Trp/Ala mutations within the membrane-penetration loops of syt strongly affect syt's ability to engage t-SNAREs in response to Ca2+, as assessed using co-flotation assays with t-SNARE-bearing, PS-free vesicles. Trp mutants (C2A2W-C2B2W, C2A3W -C2B3W) co-floated more avidly than WT C2A-C2B, while Ala mutant (C2A2A -C2B2A) exhibited diminished binding. (D) Gels in panel C were quantified by densitometry, and molar ratios of syts and syx were plotted against log[Ca2+]. Error bars represent the SEM (n ≥ 3).
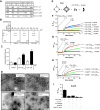
Figure 4. A tubulation-deficient syt mutant regulates the fusion of small, but not large, liposomes
(A) Syt-stimulated fusion of v-SNARE vesicles, containing donor and acceptor FRET pairs, with unlabeled t-SNARE vesicles leads to an increase in FRET-donor fluorescence. Fusion assays were carried out with either GUVs or SUVs. (B) Representative fluorescence microscopy (left) and electron microscopy (right) images of vesicles used in this study. Scale bars: 8 μm (left) and 200 nm (right). (C) Absolute NBD de-quenching signals for C2A-C2B/C2A-C2BCLM-stimulated fusion of GUVs (left) and SUVs (right). Addition of detergent produced a maximum fluorescence signal for normalization. (D) Comparison of the % syt stimulated fusion for WT C2A-C2B and the tubulation deficient mutant, C2A-C2BCLM, for GUV (left) and SUV (right) fusion assays. Relative to WT, the extent of C2A-C2BCLM stimulated fusion shows a clear loss-of-function in the GUV system (left) as compared to the SUV system. 1 mM Ca2+ was added at t = 2 min to activate syt and trigger fusion. (E) Extent of stimulated fusion for GUV (left) and SUV (right) systems plotted for four syt constructs. (F) Left: GUV-GUV fusion was blocked by pretreatment with botulinum neurotoxin B or by the use of a truncated form of SNAP-25, which mimicked the cleavage product of botulinum neurotoxin E, or by addition of the cytoplasmic domain of synaptobrevin (cd-syb) or the cytoplasmic domain of the t-SNARE heterodimers (cd-dimer). Right An SDS-PAGE gel showing that botulinum neurotoxin B efficiently cleaved GUV embedded syb.
Figure 5. N-BAR domain induced membrane-bending rescues C2A-C2BCLM-regulated membrane fusion
(A) Representative cosedimentation gels showing that N-BAR binds PS harboring liposomes in a Ca2+-independent manner. With increasing levels of PS, N-BAR shifted from the supernatant (s) to the pellet (p) fraction. At the same %PS, N-BAR binds more avidly to V105nm than to V252nm. (B) N-BAR does not exhibit significant t- or v-SNARE binding activity, while C2A-C2BClm and C2A-C2B bind t-SNAREs in a Ca2+-dependent manner, as assessed using coflotation assays. Protein free, t-SNARE- and v-SNARE-bearing vesicles are indicated as pf, Tr and Vr, respectively. (C) Quantification of coflotation assays. (D) Electron micrographs showing that 1 μM N-BAR tubulated liposomes composed of 100% Folch lipids or 15% PS/30% PE/55% PC, after 15 min incubation; the diameters of the tubules ranged from 30 nm to 90 nm. (E) In vitro membrane fusion assay setup. v-SNARE and t-SNARE GUVs were mixed with 10 μM C2A-C2BClm and 1 μM N-BAR. (F) Addition of N-BAR greatly enhanced both the rate and extent of Ca2+-triggered C2A-C2BCLM-stimulated GUV-GUV fusion, but had modest effects on WT C2A-C2B-stimulated fusion; in the absence of N-BAR, C2A-C2BCLM-stimulated GUV-GUV fusion was negligible (green trace). 1 mM Ca2+ was added at t = 20 min. (G) Sequential addition of 10 μM C2A-C2BCLM (t = 0), 1 mM CaCl2 (t = 15 min), and 1 μM N-BAR (t = 25 min) to GUV t-SNARE and v-SNARE vesicles, as indicated by arrows. (H) Sequential addition experiment as carried out in C, with N-BAR added at t = 0 and C2A-C2BCLM added at t = 25 min, as indicated by arrows. (I) N-BAR-rescued fusion was inhibited by the same agents used in Figure 4F.
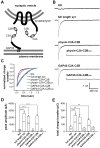
Figure 6. Synaptic vesicle and plasma membrane localized C2A-C2B both rescue rapid exocytosis in syt knock-out neurons
(A) Diagram showing the C2A-C2B fusion proteins that were expressed in syt I KO neurons. C2A-C2B was targeted to synaptic vesicles by fusing it to the C-terminus of full-length synaptophysin, or was targeted to the presynaptic plasma membrane by fusing it to the first twenty residues of GAP43. (B) Typical traces of evoked IPSCs recorded from syt I KO neurons (KO), and lentivirus infected KO neurons expressing WT syt (syt rescue), GAP43-C2A-C2B, physin-C2A-C2B, GAP43-C2A-C2BCLM or physin-C2A-C2BCLM. (C) Average normalized cumulative IPSC charge transfer over 1.5 s for KO neurons expressing the indicated constructs (D & E) Summaries of the peak amplitude (D) and total charge transfer over 1.5 s (E) of evoked IPSCs.
Figure 7. Model depicting the role of syt during Ca2+-triggered exocytosis of synaptic vesicles
(A) Before Ca2+ influx, partially assembled SNARE complexes form between the vesicle and target membrane. The two membranes are held close to each other but cannot fuse due, in part, to the clamping activity of syt, which serves to arrest _trans_-SNARE pairs (Chicka et al., 2008). Ca2+ independent interactions with t-SNARE and plasma membrane lipid PIP2 (Bai et al., 2004; Chicka et al., 2008) steer C2B toward the plasma membrane. (B) Upon Ca2+ influx, the Ca2+-binding loops of the C2B domain rapidly insert into the target membrane (Chicka et al., 2008). Insertion might be accompanied by a rotation (indicated by the curved arrow) and/or oligomerization of this domain. (C) The concerted actions of multiple C2B domains serve to locally bend the plasma membrane toward the vesicle membrane (only two C2B domains are shown in the model for simplicity). This invagination brings the two membranes into closer proximity and significantly lowers the energy barrier for bilayer merger (Martens et al., 2007; Monck and Fernandez, 1994). (D) Ca2+-syt simultaneously triggers structural transitions t-SNAREs, perhaps driving complete zippering of SNARE complexes, to initiate fusion.
Similar articles
- Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion.
Martin TF. Martin TF. Subcell Biochem. 2012;59:111-30. doi: 10.1007/978-94-007-3015-1_4. Subcell Biochem. 2012. PMID: 22374089 Free PMC article. Review. - Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion.
Bhalla A, Chicka MC, Tucker WC, Chapman ER. Bhalla A, et al. Nat Struct Mol Biol. 2006 Apr;13(4):323-30. doi: 10.1038/nsmb1076. Epub 2006 Mar 26. Nat Struct Mol Biol. 2006. PMID: 16565726 - Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion.
Lynch KL, Gerona RR, Kielar DM, Martens S, McMahon HT, Martin TF. Lynch KL, et al. Mol Biol Cell. 2008 Dec;19(12):5093-103. doi: 10.1091/mbc.e08-03-0235. Epub 2008 Sep 17. Mol Biol Cell. 2008. PMID: 18799625 Free PMC article. - Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+.
Chicka MC, Hui E, Liu H, Chapman ER. Chicka MC, et al. Nat Struct Mol Biol. 2008 Aug;15(8):827-35. doi: 10.1038/nsmb.1463. Epub 2008 Jul 11. Nat Struct Mol Biol. 2008. PMID: 18622390 Free PMC article. - How does synaptotagmin trigger neurotransmitter release?
Chapman ER. Chapman ER. Annu Rev Biochem. 2008;77:615-41. doi: 10.1146/annurev.biochem.77.062005.101135. Annu Rev Biochem. 2008. PMID: 18275379 Review.
Cited by
- Studying protein-reconstituted proteoliposome fusion with content indicators in vitro.
Diao J, Zhao M, Zhang Y, Kyoung M, Brunger AT. Diao J, et al. Bioessays. 2013 Jul;35(7):658-65. doi: 10.1002/bies.201300010. Epub 2013 Apr 29. Bioessays. 2013. PMID: 23625805 Free PMC article. - Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion.
Martin TF. Martin TF. Subcell Biochem. 2012;59:111-30. doi: 10.1007/978-94-007-3015-1_4. Subcell Biochem. 2012. PMID: 22374089 Free PMC article. Review. - Determinants of Curvature-Sensing Behavior for MARCKS-Fragment Peptides.
de Jesus AJ, White OR, Flynn AD, Yin H. de Jesus AJ, et al. Biophys J. 2016 May 10;110(9):1980-92. doi: 10.1016/j.bpj.2016.04.007. Biophys J. 2016. PMID: 27166806 Free PMC article. - Cell-cell membrane fusion induced by p15 fusion-associated small transmembrane (FAST) protein requires a novel fusion peptide motif containing a myristoylated polyproline type II helix.
Top D, Read JA, Dawe SJ, Syvitski RT, Duncan R. Top D, et al. J Biol Chem. 2012 Jan 27;287(5):3403-14. doi: 10.1074/jbc.M111.305268. Epub 2011 Dec 14. J Biol Chem. 2012. PMID: 22170056 Free PMC article. - Membrane Association and Functional Mechanism of Synaptotagmin-1 in Triggering Vesicle Fusion.
Prasad R, Zhou HX. Prasad R, et al. Biophys J. 2020 Sep 15;119(6):1255-1265. doi: 10.1016/j.bpj.2020.08.008. Epub 2020 Aug 14. Biophys J. 2020. PMID: 32882186 Free PMC article.
References
- Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. - PubMed
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nature structural & molecular biology. 2004;11:36–44. - PubMed
- Bhalla A, Chicka MC, Tucker WC, Chapman ER. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nature structural & molecular biology. 2006;13:323–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH061876-01A2/MH/NIMH NIH HHS/United States
- R01 MH061876-05A1/MH/NIMH NIH HHS/United States
- MH61876/MH/NIMH NIH HHS/United States
- R01 MH061876-02/MH/NIMH NIH HHS/United States
- R01 MH061876-06/MH/NIMH NIH HHS/United States
- R01 MH061876-04/MH/NIMH NIH HHS/United States
- R01 MH061876/MH/NIMH NIH HHS/United States
- R01 MH061876-07/MH/NIMH NIH HHS/United States
- R01 MH061876-03/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous