A study on the interactions between heparan sulfate proteoglycans and Wnt proteins - PubMed (original) (raw)
A study on the interactions between heparan sulfate proteoglycans and Wnt proteins
Christophe Fuerer et al. Dev Dyn. 2010 Jan.
Abstract
The Wnt signaling pathway plays key roles in development and adult homeostasis. Wnt proteins are secreted, lipid-modified glycoproteins. They can form morphogen gradients that are regulated at the level of protein secretion, diffusion, and internalization. These gradients can only exist if the hydrophobic Wnt proteins are prevented from aggregating in the extracellular environment. Heparan sulfate proteoglycans (HSPGs) are necessary for proper activity of Wnt proteins and influence their distribution along the morphogenetic gradient. In this study, we show that HSPGs are able to maintain the solubility of Wnt proteins, thus stabilizing their signaling activity. Our results suggest that the role of HSPGs is not only to concentrate Wnt molecules at the cell surface but also to prevent them from aggregating in the extracellular environment.
(c) 2009 Wiley-Liss, Inc.
Figures
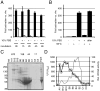
FIGURE 1. High-molecular weight fractions of fetal bovine serum stabilize Wnt activity
(A) Wnt3A proteins loose their signaling activity in serum-free conditions. Wnt3A proteins were incubated in DMEM either in absence (−) or in presence (+) of 10% FBS for the indicated times. Wnt3A activity was then assayed on LS/L reporter cells. (B) The stabilizing activity of serum is heat-labile. DMEM with (+) or without (−) 10% FBS was heated at 95°C for 10 minutes and equilibrated to 37°C before addition of Wnt3A protein. The solutions were incubated for four hours and analyzed on LS/L cells. “after”: DMEM was heated at 95°C for 10 minutes and equilibrated at 37°C before addition of 10% FBS and Wnt3A proteins. (C) Size fractionation profile of FBS as analyzed by coomassie staining of an SDS-PAGE gel. The numbers above the gel represent the molecular weight of protein standards after gel filtration chromatography as well as the corresponding fractions (A9-C5). The molecular weight markers for the gel are on the left side. All molecular weights are in kD. (D) Comparison between the Absorbance at 280nm (gray line) and the Wnt3A-sustaining activity (black line with circles) of the FBS fractions. Wnt3A proteins were incubated in the various fractions for 1 hour and assayed on LS/L cells.
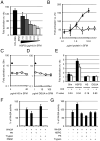
FIGURE 2. HSPGs stabilize Wnt3A activity in serum-free conditions
(A) Wnt3A proteins were incubated for one hour with complete medium (10% FBS), DMEM (SFM), or DMEM containing the indicated amount of HSPG and assayed on LS/L cells. (B) BSA does not stabilize Wnt3A. Wnt3A proteins were incubated with HSPGs (solid line, black triangles) or BSA (dashed line, white diamonds) for four hours and assayed on LS/L cells. Heparan sulfate (C) or D-glucuronic acid (D), two components of HSPGs, do not stabilize Wnt3A. Wnt3A proteins were incubated with HSPGs (triangle) or either heparan sulfate (C) or D-glucuronic acid (D) (both in solid line, white squares) for four hours and assayed on LS/L cells. (E) The stabilization activity of HSPG is sensitive to Heparitinase III. Wnt3A proteins were incubated for four hours in DMEM (alone or supplemented with 10 μg/mL HSPGs, 5 μg/mL HS, or 1.4 μg/mL DGUA) in absence (−) or presence (+) of Heparitinase III and assayed on LS/L cells. P values are calculated using the Student’s t-Test (two-tailed, homoscedastic). (F) Wnt3A activity is not impaired by Proteinase K (PK) or trypsin in presence of PMSF. Trypsin or PK were incubated for one hour, then added to Wnt3A solutions in absence or presence of PMSF, incubated for one hour, and assayed on LS/L cells. (G) HSPG core protein is important for the stability of Wnt3a. HSPGs were initially treated with either proteinase K (PK) or trypsin. After 2 hours, PMSF was added; the reaction was incubated for 10 minutes, incubated in DMEM containing Wnt3A or vehicle for 1 hour, and assayed on LS/L cells.
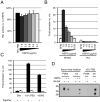
FIGURE 3. HSPGs stabilize Wnt3A activity by preventing aggregation of Wnt3A proteins
(A). HSPGs do not increase Wnt3A signaling in presence of serum. Wnt3A proteins were incubated in complete medium (10% FBS) supplemented with various amounts of HSPGs for one hour and assayed on LS/L cells. (B) HSPGs do not activate Wnt3A signaling in absence of Wnt3A protein. Wnt3A proteins or vehicle (vhc) were incubated with DMEM supplemented with various amounts of HSPGs for one hour and assayed on LS/L cells. (C) Serum or HSPGs do not act by sensitizing cells to Wnt3A. (−) Wnt3A proteins were incubated alone in DMEM for four hours and added to the same volume of DMEM (Sfm), complete medium (10% FBS), or 20 μg/ml HSPGs in DMEM (also incubated for four hours), and then assayed on LS/L cells. (+) Wnt3A proteins were incubated in DMEM, complete medium, or 10 μg/ml HSPGs in DMEM for four hours and assayed on LS/L cells. (D) HSPGs and serum act by preventing Wnt3A aggregation. Wnt3A proteins were incubated in various media for four hours at room temperature and pelleted at 16,000 × g. The pellet (protein aggregates) was resuspended in SDS-PAGE buffer while the supernatant (s/n, soluble proteins) was concentrated with blue sepharose beads and resuspended in SDS-PAGE buffer.
Similar articles
- Heparin activates Wnt signaling for neuronal morphogenesis.
Colombres M, Henríquez JP, Reig GF, Scheu J, Calderón R, Alvarez A, Brandan E, Inestrosa NC. Colombres M, et al. J Cell Physiol. 2008 Sep;216(3):805-15. doi: 10.1002/jcp.21465. J Cell Physiol. 2008. PMID: 18449906 - Roles of heparan sulfate sulfation in dentinogenesis.
Hayano S, Kurosaka H, Yanagita T, Kalus I, Milz F, Ishihara Y, Islam MN, Kawanabe N, Saito M, Kamioka H, Adachi T, Dierks T, Yamashiro T. Hayano S, et al. J Biol Chem. 2012 Apr 6;287(15):12217-29. doi: 10.1074/jbc.M111.332924. Epub 2012 Feb 20. J Biol Chem. 2012. PMID: 22351753 Free PMC article. - Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis.
Matsuo I, Kimura-Yoshida C. Matsuo I, et al. Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130545. doi: 10.1098/rstb.2013.0545. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349453 Free PMC article. Review. - Functions of heparan sulfate proteoglycans in cell signaling during development.
Lin X. Lin X. Development. 2004 Dec;131(24):6009-21. doi: 10.1242/dev.01522. Development. 2004. PMID: 15563523 Review. - Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis.
Ohkawara B, Yamamoto TS, Tada M, Ueno N. Ohkawara B, et al. Development. 2003 May;130(10):2129-38. doi: 10.1242/dev.00435. Development. 2003. PMID: 12668627
Cited by
- Matrix remodeling maintains embryonic stem cell self-renewal by activating Stat3.
Przybyla LM, Theunissen TW, Jaenisch R, Voldman J. Przybyla LM, et al. Stem Cells. 2013 Jun;31(6):1097-106. doi: 10.1002/stem.1360. Stem Cells. 2013. PMID: 23404867 Free PMC article. - Wnt5b-associated exosomes promote cancer cell migration and proliferation.
Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, Kikuchi A. Harada T, et al. Cancer Sci. 2017 Jan;108(1):42-52. doi: 10.1111/cas.13109. Epub 2016 Dec 23. Cancer Sci. 2017. PMID: 27762090 Free PMC article. - Coreceptor functions of cell surface heparan sulfate proteoglycans.
Hayashida K, Aquino RS, Park PW. Hayashida K, et al. Am J Physiol Cell Physiol. 2022 May 1;322(5):C896-C912. doi: 10.1152/ajpcell.00050.2022. Epub 2022 Mar 23. Am J Physiol Cell Physiol. 2022. PMID: 35319900 Free PMC article. Review. - Lipid-mediated Wnt protein stabilization enables serum-free culture of human organ stem cells.
Tüysüz N, van Bloois L, van den Brink S, Begthel H, Verstegen MM, Cruz LJ, Hui L, van der Laan LJ, de Jonge J, Vries R, Braakman E, Mastrobattista E, Cornelissen JJ, Clevers H, Ten Berge D. Tüysüz N, et al. Nat Commun. 2017 Mar 6;8:14578. doi: 10.1038/ncomms14578. Nat Commun. 2017. PMID: 28262686 Free PMC article. - Wnt ligand presentation and reception: from the stem cell niche to tissue engineering.
Mills KM, Szczerkowski JLA, Habib SJ. Mills KM, et al. Open Biol. 2017 Aug;7(8):170140. doi: 10.1098/rsob.170140. Open Biol. 2017. PMID: 28814649 Free PMC article. Review.
References
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. - PubMed
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. - PubMed
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, 3rd, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104:14700–14705. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources